Press release
Global Movement Disorder Market Scope by Application, Trends to Expand Significantly Upto 2026
Fact.MR has been actively involved in offering comprehensive research data concerning various topics which are associated to industrial reference and investor utility. This particular research report titled “Movement Disorder Market Forecast, Trend Analysis & Competition Tracking - Global Review 2017 to 2026” has been added to the wide online database of Fact.MR which discusses the present as well as future market scenario. Readers can access knowledge related to market volume, regional expanse as well as competitive landscape prevailing in the global movement disorder market. In order to study development patterns, this smart assessment also focuses on market dynamics, which talks about drivers, restraints and opportunities estimated to influence the concerned market during the period until 2017 to 2022.Request For Sample Report- https://www.factmr.com/connectus/sample?flag=S&rep_id=329
Key Factors Fuelling Global Market Growth
Growth of the global movement disorders market is projected to be bound by various macro-economic and micro-economic factors. Lack of ability to move due to growing prevalence of factors such as cerebrovascular diseases, trauma, brain tumors, degenerative diseases and convulsive diseases continues to impact the global market growth of movement disorder positively. Prevalence of neurological disorders negatively affect the cognitive abilities and lead to depression, incapability to chew, swallowing, speaking and insomnia. Growing awareness regarding the prevalence of various mental diseases will further contribute towards the global market growth of movement disorder significantly.
Treatments and medications that have received an approval from FDA will further impact the global market growth of movement disorders positively. Ingrezza capsules and Xadago (safinamide) tablets are two medicines that have received an approval from FDA recently. Xadago (safinamide) tablets has been approved for the treatment of Parkinson’s disease and Ingrezza capsules has been approved for the treatment of dyskinesia. Moreover, FDA has approved brain transplantation in order to reduce various symptoms of tremor. Such factors continue to impact the global market growth of movement disorder significantly.
Manufacturers in the global market are increasingly concentrating on product development and innovations in order to gain an advantage over the other market players. With the growing demand for improved results and fast recovery, manufacturers are also focusing on integrating advanced technological developments. Companies in the global market of movement disorder are offering technologically enhanced spork, fork, everyday spoon and soup spoons. Attributed to such factors, the global market of movement disorders is projected to represent significant growth throughout the forecast period.
Browse Full Report With TOC- https://www.factmr.com/report/329/movement-disorder-market
DA Approval to Boost Sales of Medications
Medications and treatments approved by FDA is also expected to impact growth of the global movement disorder market. Xadago (safinamide) tablets and Ingrezza capsules are two drugs that have recently cleared the FDA pipeline. Ingrezza capsules have recently received approval for treatment of dyskinesia and Xadago (safinamide) tablets have received approval of Parkinson’s disease. In addition, the FDA has also approved implantation of brain to reduce the tremor symptoms. These factors are further expected to boost growth of the global movement disorders market.
Advanced Features to Impact Growth of the Global Market
In order to gain a competitive edge, various companies operating in the global movement disorder market are focusing on product innovation and developments. Manufacturers are mainly concentrating on incorporating advanced technology in the medical devices for faster recovery and improved results. Equipped with the advanced features such as sensors, 360-degree stabilizing solutions and cloud technology. Parkinson’s spoon equipped with advanced technological features enable the end users to retrieve and store information regarding the status of the unwanted tremors and generates the algorithm of tremor patterns for optimal stabilization. Moreover, the manufacturers operating in the global market are offering the electronic stabilizing handle, which comprises everyday spoon, spork, soup spoon and a fork. Bound to these factors, the global movement disorders market is expected to witness significant growth during the forecast period.
On the other hand, various factors continue to impact growth of the global movement disorders market negatively. Lack of investment in research and development will continue to inhibit growth of the global movement disorder market. In addition, slow approval of the medications developed is further likely to impact growth of the global market negatively. Moreover, poor method of therapeutics conducted continues to pose significant challenges towards growth of the global market.
Parkinson ’s disease to Represent a Leading Segment
Growing need for medical devices with the uninterrupted functioning for treatment of the neurological disorders has led to an upsurge in adoption of rechargeable deep brain stimulator devices. On the basis of product type, the rechargeable deep brain stimulator devices segment is expected to generate significant revenues, accounting for a value of over US$ 500 Mn by the end of 2026. However, the non-rechargeable deep brain stimulator devices product type segment is expected to register the highest CAGR during the forecast period.
Based on end user, the hospitals segment is expected to represent the highest revenue growth, recording a value of less than US$ 100 Mn by the end of 2017. On the other hand, the clinics end user segment is expected to register a robust through 2026.
Continued@@@
Check For Discount On This Report- https://www.factmr.com/connectus/sample?flag=D&rep_id=329
About Fact.MR
Fact.MR is a fast-growing market research firm that offers the most comprehensive suite of syndicated and customized market research reports. We believe transformative intelligence can educate and inspire businesses to make smarter decisions. We know the limitations of the one-size-fits-all approach; that’s why we publish multi-industry global, regional, and country-specific research reports.
Contact Us
Fact.MR
Suite 9884
27 Upper Pembroke Street,
Dublin 2, Ireland
Telephone: +353-1-6111-593
Email: sales@factmr.com/
Web: https://www.factmr.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Movement Disorder Market Scope by Application, Trends to Expand Significantly Upto 2026 here
News-ID: 1002732 • Views: …
More Releases from Fact.MR
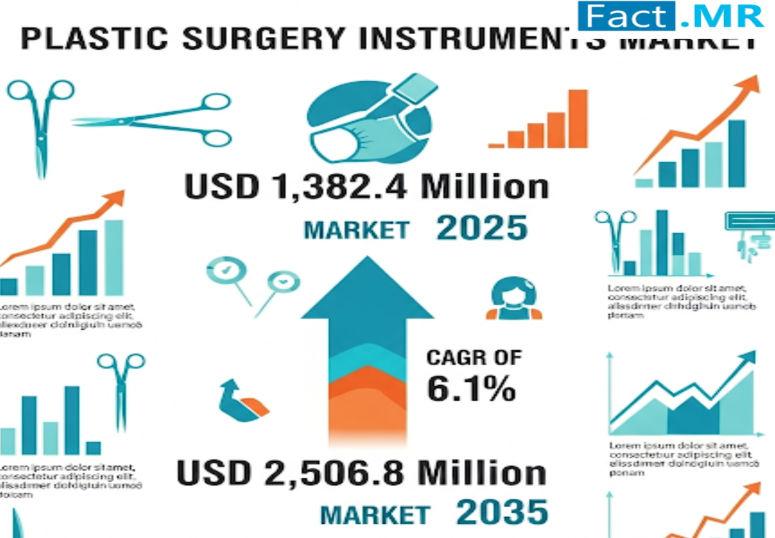
Plastic Surgery Instruments Market Valued at USD 1,382.4 Million in 2025
Plastic Surgery Instruments Market is witnessing robust growth, supported by rising demand for both cosmetic and reconstructive surgeries. According to Fact.MR's latest report, the market is valued at USD 1,382.4 million in 2025, highlighting the critical role of specialized instruments in enhancing surgical precision and patient outcomes. The popularity of aesthetic enhancements, combined with the need for reconstructive solutions in trauma and medical cases, is fueling steady adoption worldwide.
For More…
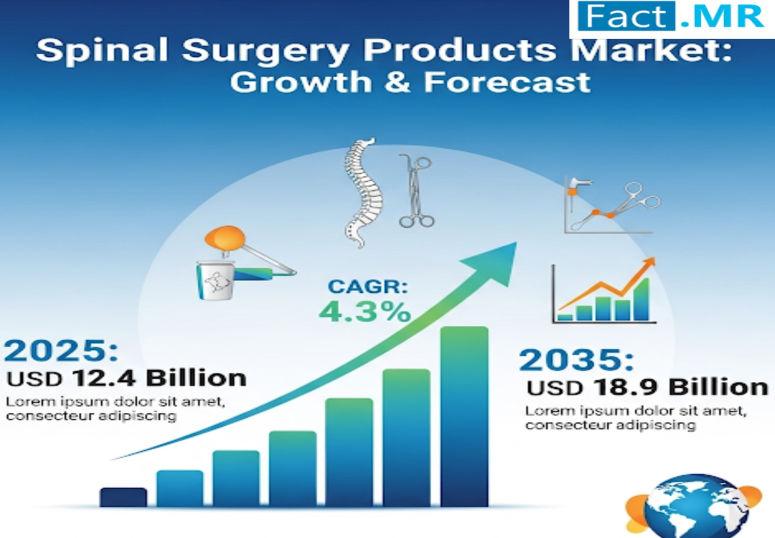
Spinal Surgery Products Market Expanding at 4.3% CAGR by 2035 | Key Players Cove …
The global spinal surgery products market continues to expand as demand for effective treatment options for spinal disorders increases. According to a recent analysis by Fact.MR, the market is valued at USD 12.4 billion in 2025, underscoring its vital role in the global healthcare industry. This growth is being driven by rising incidences of degenerative disc diseases, trauma cases, and spinal deformities, which are fueling the demand for advanced devices…
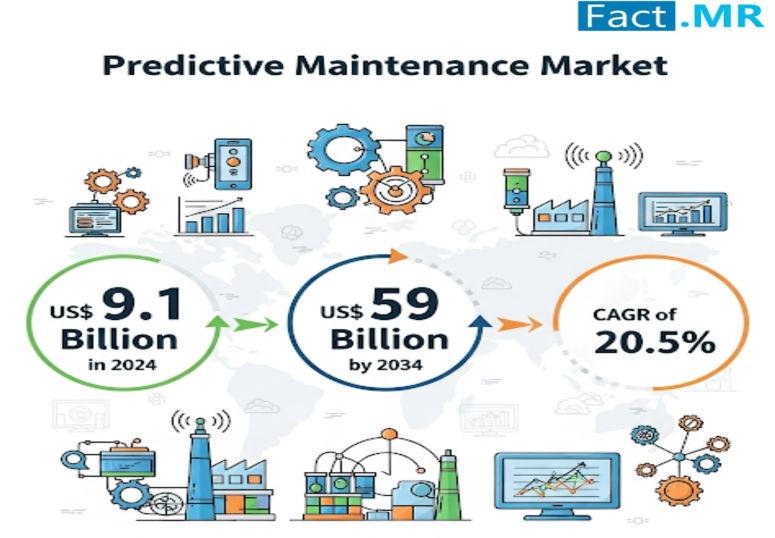
Predictive Maintenance Market Growing at 20.5% CAGR by 2034 | Fact.MR Analysis
The predictive maintenance market is witnessing rapid growth as industries across the globe increasingly rely on real-time data analytics to prevent costly equipment failures and improve operational efficiency. Predictive maintenance solutions, powered by advanced technologies such as artificial intelligence, IoT, and machine learning, enable organizations to monitor asset health, anticipate breakdowns, and optimize maintenance schedules.
According to Fact.MR, the global predictive maintenance market is valued at US$ 9.1 billion in…
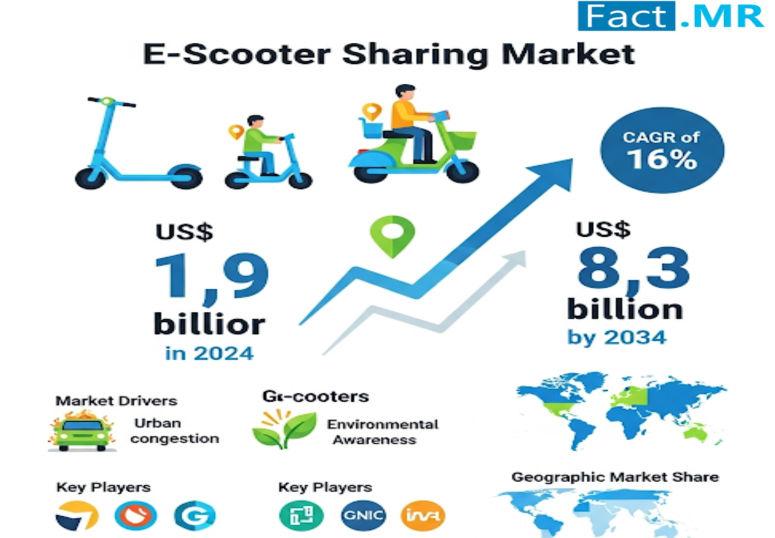
E-Scooter Sharing Market is Forecasted to Reach US$ 8.3 Billion by 2034 | Key Pl …
The global e-scooter sharing market is gaining strong momentum as cities worldwide adopt sustainable and cost-efficient mobility solutions. With urban congestion, rising fuel prices, and growing environmental concerns, e-scooter sharing services are becoming a preferred choice for short-distance commutes. According to the latest insights from Fact.MR, the global e-scooter sharing market has been analyzed at a value of US$ 1.9 billion in 2024, reflecting the increasing accessibility of shared micro-mobility…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
