Press release
GMP Cell Banking Service Market value is projected to reach US$ 3,123.5 million by 2034
According to Fact.MR, a leading provider of market research and competitive intelligence, the global GMP Cell Banking Service Market is anticipated to reach approximately US$ 882.1 million by 2024. Over the period from 2024 to 2034, the market for GMP cell banking services is expected to witness a robust growth rate of 13.7%. The report further forecasts that the market will attain an overall valuation of US$ 3,123.5 million by the conclusion of this forecast period.GMP cell banking services play a crucial role in ensuring the quality, purity, and traceability of cell lines utilized in the research and development of new treatments. Moreover, these services mitigate the risk of contamination and fluctuations in drug prices by ensuring consistency and quality in cell lines.
For More Insights into the Market, Request a Sample of this Report:
https://www.factmr.com/connectus/sample?flag=S&rep_id=9248
Top Key Players
WuXi AppTec, Charles River Laboratories, Eurofins Scientific, Merck KGaA, Lonza, SGS Life Sciences Ltd., ViruSure, Austrianova, Goodwin Biotechnology, Paragon Bioservices, Bio Reliance, Sartorius, Thermo Fisher, BSL Bioservice, Cleancells, Covance
Market Segmentations
By Cell Type: Mammalian, Microbial, Insect, Yeast, Avian, Stem, Others
By End User: Biopharmaceutical Companies, Contract Manufacturing Organizations
By Region: North America, Europe, Latin America, East Asia, South Asia & Oceania, The Middle East & Africa
The size and competitiveness of different GMP cell line end users are anticipated to determine the future growth prospects for the market under discussion. Companies that can deploy modern equipment to produce and preserve cell lines are expected to adapt to the ever-changing GMP cell banking services market.
Key Takeaways from the Global GMP Cell Banking Service Market Study Report
The United States is the leading market for GMP cell banking services, which is expected to advance with a 13.1% annual growth rate till 2034.
Canada is estimated to have around 22.8% of the North American market and is projected to follow 12.7% CAGR through 2034.
In Europe, Germany is observed to have around 33.4% of the total demand for GMP cell banking services. The regional market is projected to expand further at 12% per year between 2024 and 2034.
The adoption of GMP cell banking services in the United Kingdom spurred after the pandemic, and it is likely to experience a 10.7% CAGR through 2034.
Competitive Landscape
A few leading players hold a significant share of the global market and offer complete GMP-compliant services, encompassing characterization, testing, and storage. Businesses that outsource cell banking services also look for partners who have experience, resources, and a history of adhering to GMP regulations.
Recent Developments
In September 2022, FUJIFILM Diosynth Biotechnologies revealed that it was expanding its large-scale microbial production facility at the Billingham campus located in the United Kingdom.
In April 2022, SGS collaborated with Liveome, a drug developer based in Korea, to formulate, develop, and clinically manufacture a targeted-release formulation containing lyophilized microbial cells.
Get Customization on this Report for Specific Research Solutions:
https://www.factmr.com/connectus/sample?flag=RC&rep_id=9248
Biopharmaceutical Companies Increasingly Rely on Outsourcing to CMOs
According to market analysis, there is a projected 13.9% Compound Annual Growth Rate (CAGR) in the demand for GMP cell banking services by contract manufacturing companies through 2034.
The current trend in the biopharmaceutical industry involves outsourcing specific phases of the production process to contract manufacturing organizations (CMOs). Biopharmaceutical companies find that collaborating with CMOs not only provides access to infrastructure and expertise at a reasonable cost but also proves to be a more efficient alternative to developing internal cell line banking capabilities, which can be resource-intensive.
At the Core: Efficiency and Quality Assurance Requirements of Biopharmaceutical Companies
A survey across various end-use verticals for GMP cell banking service providers reveals that biopharmaceutical companies contribute to over 70% of the total demand. The analysis suggests a CAGR of 13.3% for this segment between 2024 and 2034.
GMP cell banking services play a crucial role for biopharmaceutical companies in ensuring the consistency, quality, and legal compliance of the cell lines used in developing new drugs. Additionally, these services streamline the time required for the development and commercialization of treatments by providing ready-to-use and compliant cell banks.
About Fact.MR
Market research and consulting agency with a difference! That's why 80% of Fortune 1,000 companies trust us for making their most critical decisions. While our experienced consultants employ the latest technologies to extract hard-to-find insights, we believe our USP is the trust clients have on our expertise. Spanning a wide range - from automotive & industry 4.0 to healthcare & retail, our coverage is expansive, but we ensure even the most niche categories are analyzed. Our sales offices in United States and Dublin, Ireland. Headquarter based in Dubai, UAE. Reach out to us with your goals, and we'll be an able research partner.
Contact:
US Sales Office :
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail: sales@factmr.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Cell Banking Service Market value is projected to reach US$ 3,123.5 million by 2034 here
News-ID: 3473060 • Views: …
More Releases from Fact.MR

Managed Security Services Market is Projected to Reach USD 93.72 Billion by 2034 …
The global managed security services (MSS) market is estimated at USD 27.85 billion in 2024 and is projected to increase at a remarkable CAGR of 12.9% to reach a value of USD 93.72 billion by the end of 2034. This expansion is driven by the escalating cyber threats, regulatory compliance requirements, and the growing complexity of IT environments. Managed security services provide outsourced monitoring and management of security devices and…
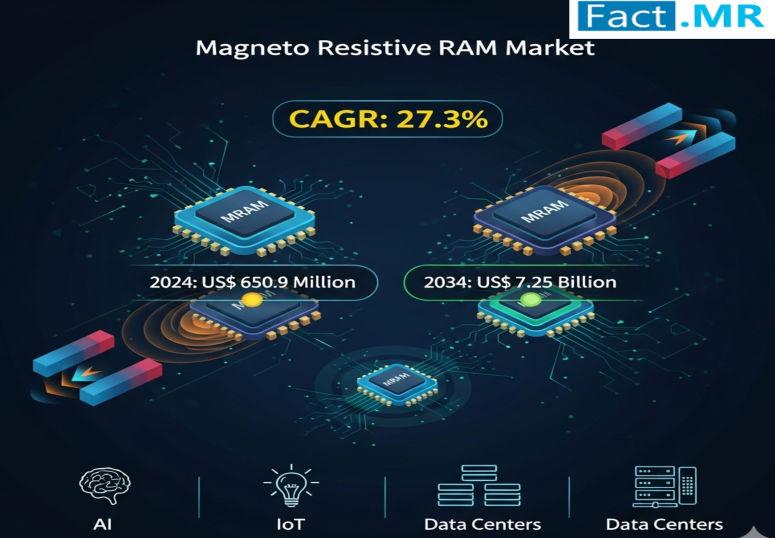
Magneto Resistive RAM Market is Estimated to Reach USD 7.25 Billion by 2034 | Ke …
Magneto Resistive RAM Market is estimated at USD 650.9 million in 2024 and is projected to grow at a CAGR of 27.3%, reaching USD 7.25 billion by 2034. This explosive expansion is driven by MRAM's superior attributes, including high-speed access, compact size, and resilience, making it ideal for edge computing, IoT devices, and applications requiring data durability. MRAM is increasingly replacing traditional flash and DRAM due to its scalability, density,…

Scalp Health Market is Forecasted to Reach USD 18.4 Billion by 2035 | Fact.MR Re …
Scalp Health Market is projected to reach USD 18.4 billion by 2035, rising from USD 9.6 billion in 2025, with a projected CAGR of 6.7% during the forecast period. This shift signifies a critical evolution in consumer behavior - one where the scalp is no longer viewed as a peripheral concern within haircare but as a standalone health domain intersecting dermatology, neurocosmetics, and preventative wellness. Driven by rising awareness of…
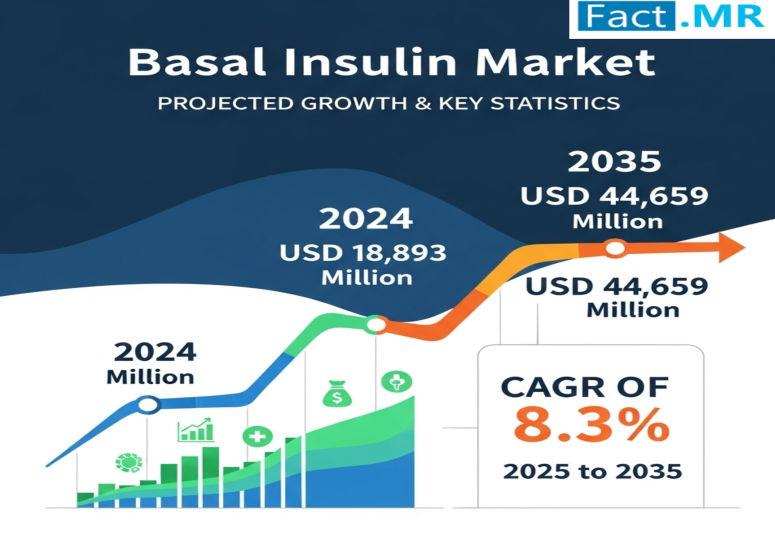
Basal Insulin Market is Projected to Reach USD 44,659 Million by 2035 | Key Play …
The global basal insulin market is expected to reach USD 44,659 million by 2035, up from USD 18,893 million in 2024. During the forecast period 2025 to 2035, the industry is projected to register at a CAGR of 8.3%, driven by the increasing burden of Type 2 diabetes, patient-friendly once-daily dosing, and advancements in long-acting analogs such as degludec and glargine. Enhanced delivery technologies, robust R&D, and expanding global diabetes…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…