Press release
Hereditary Angioedema Drug Pipeline Analysis, Key Developments, Trends, and Market Outlook & Market Insights for|2024-2032
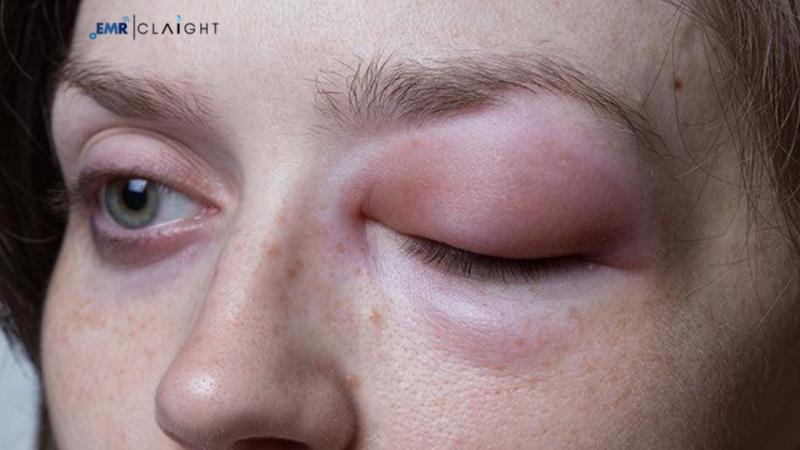
According to the Rare Disease Advisor, the prevalence of hereditary angioedema (HAE) is estimated to be between 1 in 10,000 and 1 in 50,000. Every year, HAE episodes cause 15,000 to 30,000 visits to emergency rooms in the United States. Hereditary Angioedema Drug Pipeline Analysis It makes up around 2% of all cases of clinical angioedema, which afflicts about 20% of the population. Several companies are engaged in research initiatives to address the need for effective treatment for the rare disorder. The growing focus on better therapeutic solutions for HAE has led to the development of a robust drug pipeline, offering hope for better management and care.Get a Free Sample Report with a Table of Contents: https://tinyurl.com/252ctqhh
Hereditary Angioedema Drug Pipeline Analysis Overview
Hereditary Angioedema (HAE) is a genetic condition that results in recurrent, severe swelling in various parts of the body, including the extremities, gastrointestinal tract, and upper respiratory tract. These episodes of swelling can be extremely painful and, in some cases, life-threatening. The genetic mutations leading to HAE typically affect the C1 inhibitor (C1-INH) protein, which regulates inflammation by inhibiting the activation of the complement system.
The current treatment landscape for HAE primarily revolves around managing acute attacks, preventing swelling, and improving the quality of life for patients. While some therapies are already available, there is still an unmet need for more effective, convenient, and long-lasting treatment options.
The Hereditary Angioedema drug pipeline consists of various therapeutic approaches that include targeted therapies, biologics, and gene-editing technologies. These innovations are being developed to provide long-term relief and reduce the frequency and severity of HAE attacks.
Hereditary Angioedema Drug Pipeline Analysis Dynamics
The dynamics of the HAE drug pipeline are shaped by several factors, including advancements in gene therapy, the growing understanding of HAE pathophysiology, regulatory approvals, and market demand for effective treatments. Key drivers in this space include:
Increasing Prevalence of HAE: As awareness of HAE grows and diagnostic capabilities improve, the number of diagnosed cases is expected to rise, leading to greater demand for treatment options.
Increased Focus on Targeted Treatments: Unlike traditional therapies, targeted treatments aim to address the underlying genetic and molecular causes of HAE. This focus on precision medicine is driving innovation in the pipeline.
Gene Therapy and CRISPR Technology: The use of gene-editing technologies, such as CRISPR, offers the potential to modify the genetic mutations responsible for HAE, providing a long-term cure rather than just symptomatic relief.
Read Full Report with Table of Contents: https://tinyurl.com/23o9fm2y
Regulatory and Clinical Advancements: Recent advancements in clinical trials, as well as positive regulatory approvals for new therapies, are accelerating the development of drugs in the HAE pipeline.
Increased Investment in R&D: With major pharmaceutical companies showing increased interest in rare diseases, investment in HAE research and development is rising, which should result in more diverse treatment options.
External Hereditary Angioedema Drug Pipeline Analysis Trends
Several external factors influence the Hereditary Angioedema drug pipeline analysis:
Regulatory Trends: Regulatory agencies like the FDA and EMA are prioritizing orphan drugs, speeding up approvals for treatments that address rare conditions like HAE. This trend is expected to continue, enabling quicker access to new therapies.
Technological Advancements: The growing interest in gene editing, artificial intelligence, and personalised medicine is opening up new opportunities for developing more effective treatments for HAE.
Collaborations and Partnerships: Pharmaceutical companies are increasingly forming partnerships with biotechnology firms to advance the development of HAE therapies. These collaborations bring together resources, expertise, and technology to push forward innovative treatments.
Patient Advocacy: As patient advocacy groups continue to raise awareness of HAE, there is growing pressure on companies and regulatory bodies to fast-track the approval of new therapies.
Healthcare Reimbursement Policies: The approval of more advanced therapies, including biologics and gene therapies, may encounter hurdles related to cost-effectiveness and reimbursement, affecting patient access.
Hereditary Angioedema Drug Pipeline Analysis Segmentation
The HAE drug pipeline can be segmented based on several factors, including drug type, stage of development, mechanism of action, and therapy type.
By Drug Type:
C1 Inhibitor Replacement Therapies: These therapies aim to replenish the deficient or dysfunctional C1-INH protein, which is central to HAE.
Bradykinin B2 Receptor Antagonists: These drugs block the action of bradykinin, a peptide that causes vasodilation and contributes to the swelling seen in HAE attacks.
Gene Therapy: Focused on correcting the genetic mutations that lead to HAE, these therapies represent a potential long-term solution.
By Stage of Development:
Preclinical Stage: New drugs that are in the initial phases of development, undergoing laboratory studies.
Phase I: Early trials testing safety and tolerability in healthy volunteers or small groups of patients.
Phase II & III: Clinical trials focused on efficacy, dosing, and side effects.
Approved: Drugs that have received approval for clinical use by regulatory authorities.
By Mechanism of Action:
Preventive Therapies: Designed to reduce the frequency and severity of HAE attacks.
Acute Attack Therapies: Used to treat or alleviate symptoms during an acute episode of swelling.
By Therapy Type:
Monoclonal Antibodies: These biologics are designed to target specific pathways involved in the pathophysiology of HAE.
Small Molecule Drugs: Chemical compounds targeting various aspects of the disease process.
Gene Therapy: Novel therapies that aim to correct the genetic root cause of HAE.
Hereditary Angioedema Drug Pipeline Analysis Growth
The growth of the HAE drug pipeline can be attributed to several key factors:
Technological Innovations: As new technologies such as CRISPR and gene-editing techniques continue to evolve, they offer promising new avenues for treatment, pushing the boundaries of what's possible in HAE care.
Increased Investment: Venture capital and pharmaceutical companies are investing heavily in research to develop effective treatments for rare diseases, including HAE. This is driving the growth of the drug pipeline.
Market Demand: The rising number of HAE diagnoses and increasing awareness about the condition are creating a demand for more effective and accessible treatment options.
Clinical Trial Advancements: Positive results from clinical trials are propelling drugs further down the development pipeline, increasing optimism in the market.
Regulatory Push: Both the FDA and EMA are offering incentives like orphan drug status, fast-track designation, and priority review to encourage the development of drugs for rare diseases like HAE.
Recent Hereditary Angioedema Drug Pipeline Analysis Market
In recent years, the HAE drug pipeline has seen the introduction of several promising treatments. For example, the development of Orladeyo (berotralstat) as an oral medication to prevent HAE attacks marks a significant step forward in the management of the disease. This approval, along with others, has bolstered confidence in the pipeline's potential.
Other key treatments such as Cinryze (C1 esterase inhibitor), Haegarda, and Firazyr are widely used to manage acute HAE attacks and prevent future episodes. However, many of these treatments are still focused on alleviating symptoms rather than offering a long-term solution.
The newer gene-editing approaches under investigation are generating significant interest, as they could potentially offer a cure for patients suffering from the genetic mutations that cause HAE. The development of monoclonal antibodies is also progressing, with drugs such as Lanadelumab (Takhzyro) demonstrating promising results in clinical trials.
Hereditary Angioedema Drug Pipeline Analysis Scope
The scope of the HAE drug pipeline extends well beyond the current therapies available. New drug classes and gene therapies are poised to change the way HAE is treated, with the potential for curative treatments on the horizon. Key factors driving this expanded scope include:
Increased Patient Access: As the number of diagnosed patients rises and healthcare systems evolve, there will be greater demand for new treatments.
Expanded Indications: The approval of therapies for multiple types of HAE, including different genetic variants, will broaden the scope of treatment.
Global Reach: The market for HAE drugs is not confined to the U.S. and Europe. With increasing awareness in Asia and other regions, the global scope for HAE treatments is expanding.
COVID-19 Impact Analysis
The COVID-19 pandemic has had a significant impact on the healthcare and pharmaceutical industries, including the Hereditary Angioedema drug pipeline. On one hand, the pandemic caused delays in clinical trials and regulatory approvals due to resource reallocations and logistical challenges. On the other hand, the increased focus on biotechnology during the pandemic has accelerated investment in rare disease therapies, including those for HAE.
Furthermore, the pandemic's impact on healthcare systems globally has highlighted the importance of developing effective treatments for chronic and rare diseases, further fueling investment and innovation in the HAE drug pipeline.
Key Players in the Hereditary Angioedema Drug Pipeline
Several key players are leading the development of new treatments for HAE, including:
Astria Therapeutics, Inc.: Known for their work on innovative therapies for rare diseases, Astria Therapeutics is developing treatments aimed at improving the quality of life for HAE patients.
Ionis Pharmaceuticals, Inc.: A leader in RNA-targeted therapeutics, Ionis is focused on developing drugs that can target specific genes involved in HAE pathophysiology.
Intellia Therapeutics: With its gene-editing approach, Intellia is at the forefront of exploring CRISPR technologies to offer potential cures for genetic disorders, including HAE.
FAQ
Q1: What is the cause of Hereditary Angioedema?
Hereditary Angioedema is caused by genetic mutations that lead to a deficiency or dysfunction of the C1-INH protein, which regulates the complement system and inflammation.
Q2: How is HAE diagnosed?
HAE is diagnosed through a combination of clinical symptoms and laboratory tests, including measuring C1-INH levels and functional activity.
Q3: What are the current treatment options for HAE?
Current treatments include C1 inhibitor replacement therapies, bradykinin receptor antagonists, and therapies for acute attacks, such as intravenous or subcutaneous medications.
Q4: What are the future prospects for HAE treatments?
The future of HAE treatments lies in gene therapy, monoclonal antibodies, and CRISPR-based technologies that could provide long-term relief or a cure.
Q5: Which companies are leading the HAE drug pipeline?
Key players include Astria Therapeutics, Ionis Pharmaceuticals, and Intellia Therapeutics, all of which are focused on advancing new treatments for HAE.
Human Papillomavirus (HPV) Drug Pipeline Analysis:https://tinyurl.com/2alvrv3h
Gastric Neuroendocrine Tumors Drug Pipeline Analysis: https://tinyurl.com/23ngm2ax
Metastatic Prostate Cancer Drug Pipeline Analysis: https://tinyurl.com/27gyc75s
Steroid Refractory Acute Graft Versus Host Disease Drug Pipeline Analysis: https://tinyurl.com/2bqqah8a
Media Contact:
Company Name: Claight Corporation
Contact Person: James William, Corporate Sales Specialist - U.S.A.
Email: sales@expertmarketresearch.com
Toll Free Number: +1-415-325-5166 | +44-702-402-5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com
Aus Site: https://www.expertmarketresearch.com.au
Acquire unparalleled access to critical industry insights with our comprehensive market research reports, meticulously prepared by a team of seasoned experts. These reports are designed to equip decision-makers with an in-depth understanding of prevailing market trends, competitive landscapes, and growth opportunities.
Our high-quality, data-driven analysis provides the essential framework for organisations seeking to make informed and strategic decisions in an increasingly complex and rapidly evolving business environment. By investing in our market research reports, you can ensure your organisation remains agile, proactive, and poised for success in today's competitive market.
Don't miss the opportunity to elevate your business intelligence and strengthen your strategic planning. Secure your organisation's future success by acquiring one of our Expert Market Research reports today.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Hereditary Angioedema Drug Pipeline Analysis, Key Developments, Trends, and Market Outlook & Market Insights for|2024-2032 here
News-ID: 3788077 • Views: …
More Releases from Expert Market Research

Exploring the Medical Biomimetics Market: Innovations Inspired by Nature
The Medical Biomimetics Market represents one of the most transformative intersections of biology, engineering, and healthcare innovation. Biomimetics-also known as biomimicry-refers to the practice of studying nature's designs, processes, and systems to solve complex human challenges. When applied to medicine, these natural inspirations translate into advanced materials, devices, and therapeutic approaches that mimic the structure, function, or behavior of biological organisms. As healthcare systems strive for greater precision, faster healing,…
Lime Market Trends and Forecast: Growing at a Robust CAGR of 5.90% from 2025 to …
Lime Market: A Critical Industry for Future Growth
The lime market plays a vital role in various industrial processes across the globe, especially in sectors like construction, agriculture, steel manufacturing, and environmental management. As industries expand and diversify, the demand for lime, an essential mineral, continues to grow, fueling the market's development. According to a recent report from Expert Market Research (EMR), the global lime market reached a volume of nearly…

Chocolate Spread Market: Key Trends, Insights by 2034
The chocolate spread market has seen steady growth in recent years, with consumer preferences shifting towards more diverse, flavorful, and ethically sourced products. Valued at USD 4.38 billion in 2024, the market is expected to grow at a CAGR of 5.70% from 2025 to 2034, reaching a projected USD 7.62 billion by 2034. This growth is driven by an increasing focus on sustainable production practices, the growing popularity of various…

Mushroom Market Size, Share and Growth Forecast (2025-2034)
Mushroom Market Outlook
According to the report by Expert Market Research (EMR), the global mushroom market attained a value of USD 68.03 billion in 2024. Driven by the increasing demand for healthy and functional foods, as well as the rising applications of mushrooms across the food, pharmaceutical, and nutraceutical industries, the market is projected to grow at a robust compound annual growth rate (CAGR) of 8.00% between 2025 and 2034, reaching…
More Releases for HAE
Hereditary Angioedema (HAE) market is expected to reach USD 4.2 billion by 2034
Hereditary Angioedema (HAE) is a rare, potentially life-threatening genetic disorder characterized by recurrent swelling episodes in the skin, gastrointestinal tract, and airways. Caused by C1 inhibitor deficiency or dysfunction, HAE affects an estimated 1 in 50,000 people worldwide. Though once underdiagnosed and undertreated, awareness and therapeutic innovation have dramatically changed the disease landscape in recent years.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/71655
The HAE market has witnessed a…
Hereditary Angioedema (HAE) Treatment Market Analysis Report 2022 - 2030
Acumen Research and Consulting has announced the addition of the "Hereditary Angioedema (HAE) Treatment Market" report to their offering.
The Hereditary Angioedema (HAE) Treatment Market Report 2030 is an in depth study analyzing the current state of the Hereditary Angioedema (HAE) Treatment Market. It provides brief overview of the market focusing on definitions, market segmentation, end-use applications and industry chain analysis. The study on Hereditary Angioedema (HAE) Treatment Market provides analysis…
HAE MEMBERSHIP GIVES DIAMOND BLADES BUSINESS A CUTTING EDGE
The UK construction industry brings in billions of pounds of new work every month*. The sector relies on tool and equipment hire companies to deliver the kit needed to undertake these projects, so how can suppliers gain a competitive edge in such an important marketplace?
Established in 2003, Mexco has already grown from its Cornwall base to become one of the UK and Ireland’s largest privately-owned suppliers of diamond blades, core…
MENTAL HEALTH IN THE SPOTLIGHT AT HAE EHA CONFERENCE
Members, non-members and exhibitors attending HAE EHA’s annual conference at Holywell Park in Loughborough were given an ideal opportunity to assess and improve their strategies for dealing with mental health in the workplace by expert speakers on this growing issue.
It is estimated mental health problems blight one in four of the UK population (http://www.hse.gov.uk/stress/mental-health.htm), a figure replicated last year in the construction industry according to a survey by Construction News’…
Hereditary Angioedema (HAE) - Pipeline Review, Trends, and Forecast Report 2017
Market Research Hub (MRH) has recently announced the addition of a fresh report, titled “Hereditary Angioedema (HAE) - Pipeline Review, H1 2017” to its report offerings. The report covers the descriptive pharmacological action of the therapeutics, its complete research and development history and latest news and press releases.
Request Free Sample Report: http://www.marketresearchhub.com/enquiry.php?type=S&repid=1196650
Global Markets Direct's latest Pharmaceutical and Healthcare disease pipeline guide Hereditary Angioedema (HAE) (C1 Esterase Inhibitor [C1-INH] Deficiency) -…
Hereditary Angioedema (HAE) Market Research Report | Forecast up to 2024
Global Hereditary Angioedema (HAE) Market: Overview
Hereditary angioedema (HAE) is a rare inherited disease, which leads to repeated episodes of swelling beneath the surface of the skin. Hereditary angioedema is confined to the face, tongue, feet, legs, hands and arms. However, it sometimes leads to swelling in airways such as trachea, larynx or intestinal tract may prove out to be fatal.
For the treatment of hereditary angioedema, currently there are no medications…