Press release
PD-L1 and PIK3CA Testing Product Market Grows with Rise in Precision Oncology & Targeted Cancer Therapies | CAGR of 18.45%
"The PD-L1 and PIK3CA Testing Product Market is estimated to reach at a high CAGR during the forecast period (2024-2031)." As per DataM intelligence research reportMarket growth opportunities(2025-2031):
The testing market is expanding as demand for PD‐L1 and PIK3CA biomarkers grows in oncology diagnostics and companion diagnostics. Multiplex assay platforms integrating both markers are gaining traction in trials and clinical workflows. Point-of-care and decentralized diagnostics are emerging, offering faster turn-around and broader access. Expanded precision oncology and targeted therapy approvals are fueling uptake. Adoption in emerging markets is accelerating thanks to improving diagnostics infrastructure and greater cancer awareness
Download your exclusive sample report today: (corporate email gets priority access): https://www.datamintelligence.com/download-sample/pd-l1-and-pik3ca-testing-product-market?sp
PD-L1 and PIK3CA Testing Product Market: Recent Industry Developments
✅ In April 2025, Agilent PD L1 IHC 22C3 pharmDx assay received European IVDR certification as a companion diagnostic for identifying gastric/gastroesophageal junction adenocarcinoma patients eligible for pembrolizumab (Keytruda®). It is now certified for seven cancer indications across EU diagnostics laboratories
✅ In December 2024, Agilent's PD L1 IHC 28 8 pharmDx assay secured Class C IVDR certification in Europe, expanding its diagnostic use across nine cancer types including NSCLC, urothelial carcinoma, melanoma, esophageal and gastric cancers enhancing confidence in PD L1 guided immunotherapy selection
✅ Roche's VENTANA PD L1 (SP263) Assay was FDA approved in October 2021 as a companion diagnostic to identify NSCLC patients suitable for Tecentriq® (atezolizumab) adjuvant therapy Mbased on the IMpower010 study showing reduced recurrence risk
✅ For PIK3CA mutations, Roche offers the cobas® PIK3CA Mutation Test kit (RUO), enabling real time PCR detection of mutations in exons 1, 4, 7, 9, and 20 from FFPE samples with >95 % reliability primarily for research use
✅ In October 2024, FDA approved inavolisib (Itovebi®) for PIK3CA mutant breast cancer, using FoundationOne Liquid CDx as the companion diagnostic to identify eligible patients marking a significant therapeutic and diagnostic linkage for targeted PI3K inhibitor use
PD-L1 and PIK3CA Testing Product Market: Drivers
Rapid growth in immunotherapies targeting the PD‐1/PD‐L1 axis and PI3K inhibitors is driving demand for accurate testing. Precision medicine mandates biomarker stratification for targeted treatments. Companion diagnostics integration and regulatory trial requirements bolster market relevance. Advanced molecular platforms (IHC, NGS) and industry collaborations further support demand. Standardization and quality assurance remain key to clinical trust and broader adoption
Strategic Players Driving the PD-L1 and PIK3CA Testing Product Market Forward:
F. Hoffmann-La Roche Ltd, Agilent Technologies, QIAGEN, EntroGen, Inc., Amoy Diagnostics, ACCB Biotech, Myriad Genetic Laboratories, Inc. and FOUNDATION MEDICINE, INC.
Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/pd-l1-and-pik3ca-testing-product-market?sp
Segment Covered in the PD-L1 and PIK3CA Testing Product Market:
➥By Product Type: PD-L1, Cervical Cancer, Esophageal Cancer, NSCLC-Squamous, Squamous Cell Carcinoma of the Head and Neck, Triple-Negative Breast Cancer, Urothelial Carcinoma, PIK3CA
➥By End-User: Hospital, Diagnostic Center, Cancer Center, Others
Regional Analysis for PD-L1 and PIK3CA Testing Product Market:
North America currently dominates global market share, supported by mature oncology infrastructure and strong clinical research activity. Europe takes the second‐largest share with widespread diagnostic adoption and reimbursement support. Asia‐Pacific represents the fastest-growing region, fueled by rising cancer prevalence, improving healthcare access, and expanding precision oncology awareness
Why Purchase the Report for PD-L1 and PIK3CA Testing Product Market?:
➠ Technology & Innovation: Tracks clinical trials and upcoming pharmaceutical advancements to stay ahead in product development.
➠ Market Positioning & Competitive Strategy: Analyzes product performance, market share, and competitor tactics for strategic decision-making.
➠ Real-World Evidence & Physician Insights: Incorporates patient data and physician behavior to align products with actual healthcare needs.
➠ Pricing & Market Access: Reviews reimbursement trends and access models to optimize product pricing and launch strategies.
➠ Regulatory & Industry Shifts: Covers policy changes, health system dynamics, and technology trends impacting market opportunities.
➠ Regional Growth & Expansion: Identifies high-growth markets and investment opportunities for geographic expansion.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=pd-l1-and-pik3ca-testing-product-market?sp
Conclusion:
The PD‐L1 and PIK3CA testing product market is entering a period of robust growth as precision oncology expands. Multiplex biomarker panels and decentralized testing platforms will increase accessibility and ease integration into clinical workflows. North America and Europe are current leaders, while Asia‐Pacific is poised to deliver rapid growth. Market success will rely on innovations in assay standardization, reagent performance, regulatory alignment, and partnerships with pharma and diagnostics providers
Request for 2 Days FREE Trial Access:
https://www.datamintelligence.com/reports-subscription?sp
✅ Competitive Landscape
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Consumer Behavior & Demand Analysis
✅ Import-Export Data Monitoring
✅ Live Market & Pricing Trends
Have a look at our Subscription Dashboard:
https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release PD-L1 and PIK3CA Testing Product Market Grows with Rise in Precision Oncology & Targeted Cancer Therapies | CAGR of 18.45% here
News-ID: 4131229 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
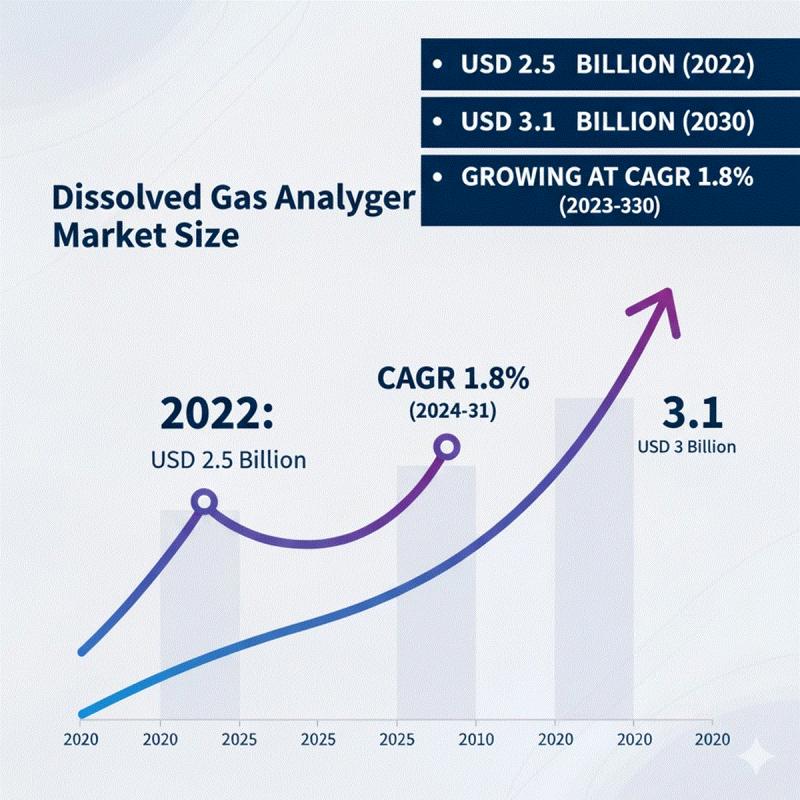
United States Dissolved Gas Analyzer Market Size, Share, Growth Drivers, Opportu …
Market Size and Growth
Leander, Texas and TOKYO, Japan - Nov. 18 2025 The market growth is primarily driven by increasing adoption of advanced monitoring and diagnostic solutions in power generation, oil & gas, and industrial sectors, where real-time detection of dissolved gases is critical for equipment reliability and safety. Rising emphasis on predictive maintenance, operational efficiency, and environmental compliance further supports market expansion. CAGR of 1.8% during the forecast…

United States Portable Power Station Market Size, Share, Trends, Latest innovati …
Leander, Texas, United States Nov.18.2025
"Global Portable Power Station Market is growing at a significant CAGR during the forecast period (2024-2031)." As per DataM intelligence research report
Download your exclusive sample report today: (corporate email gets priority access): https://www.datamintelligence.com/download-sample/portable-power-station-market?sp
United States: Recent Industry Developments
✅ In November 2025, Goal Zero launched next-generation portable power stations with higher battery capacity and fast-charging capabilities. The devices support off-grid adventures and emergency backup power. This development strengthens…

United States Monoclonal Antibody Therapeutics Market valuation $159,394.38 mill …
Leander, Texas, United States Nov.18.2025
"The Global Monoclonal Antibody Therapeutics Market reached USD 190,698.3 million in 2022 and is projected to witness lucrative growth by reaching up to USD 531,314.6 million by 2031. The global monoclonal antibody therapeutics market is expected to exhibi" As per DataM intelligence research report
Download your exclusive sample report today: (corporate email gets priority access): https://www.datamintelligence.com/download-sample/monoclonal-antibody-therapeutics-market?sp
United States: Recent Industry Developments
✅ In November 2025, Regeneron Pharmaceuticals launched new…

United States Automotive Polycarbonate Glazing Market Size, Share, Trends, Lates …
Leander, Texas, United States Nov.18.2025
"Automotive Polycarbonate Glazing Market is growing at a significant CAGR during the forecast period (2024-2031)." As per DataM intelligence research report
Download your exclusive sample report today: (corporate email gets priority access): https://www.datamintelligence.com/download-sample/automotive-polycarbonate-glazing-market?sp
United States: Recent Industry Developments
✅ In November 2025, Covestro launched advanced polycarbonate glazing solutions for automotive sunroofs and windows, enhancing impact resistance and optical clarity. The lightweight materials improve fuel efficiency while maintaining safety standards.…
More Releases for PIK3CA
PD-L1 And PIK3CA Testing Product Market Size by Type, Application, and Regional …
According to Market Research Intellect, the global PD-L1 And PIK3CA Testing Product market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The PD-L1 and PIK3CA testing product market is experiencing strong growth due to rising demand…
PD-L1 and PIK3CA Testing Product Market Comprehensive Study: Growth Outlook and …
The PD-L1 and PIK3CA Testing Product Market report by DataM Intelligence provides insights into the latest trends and developments in the market. This report identifies the key growth opportunities in the market and provides recommendations for market participants to capitalize on these opportunities. Overall, the PD-L1 and PIK3CA Testing Product market report is an essential resource for market participants who are looking to gain a comprehensive understanding of the market…
PD-L1 and PIK3CA Testing Product Market Statistical Forecast, Trade Analysis 202 …
The PD-L1 and PIK3CA testing product market is anticipated to achieve a CAGR of 18.45% by 2031.
Will the PD-L1 and PIK3CA Testing Product market emerge as the sector's next great thing? To discover the answer, look at the PD-L1 and PIK3CA Testing Product market analysis and projections. In-depth insight of the opportunities, difficulties, and trends now impacting the Machinery landscape is provided by this market research study, empowering industry participants…
PD-L1 and PIK3CA Testing Market Set to Surpass USD 2,438.4 Million by 2030 | Roc …
The latest report by Congruence Market Insights, titled 'Global PD-L1 and PIK3CA Testing Market - Size, Trends, Share, Growth, Dynamics, Competition, and Opportunity Forecast, 2023 - 2030,' provides a thorough analysis of the global PD-L1 and PIK3CA Testing market. The report meticulously examines both macro and micro trends, offering insights into the dynamic factors influencing the market. It encompasses a detailed exploration of qualitative and quantitative aspects, delivering a precise…
PD-L1 and PIK3CA Testing Product Market to Eyewitness Massive Growth by 2028 | A …
The Latest Released PD-L1 and PIK3CA Testing Product market study has evaluated the future growth potential of PD-L1 and PIK3CA Testing Product market and provides information and useful stats on market structure and size. The report is intended to provide market intelligence and strategic insights to help decision-makers take sound investment decisions and identify potential gaps and growth opportunities. Additionally, the report also identifies and analyses changing dynamics, and emerging…
PD-L1 and PIK3CA Testing Product Market Growth, Trends, Share, COVID-19 Analysis …
The global PD-L1 and PIK3CA Testing Product market size is projected to reach US$ 2396 million by 2030, from US$ 405 million in 2020, at a CAGR of 18.45% during 2021-2028
The worldwide PD-L1 and PIK3CA Testing Product market report is the very much investigated answer for the chiefs and academicians who are looking for a definite examination regarding both subjective just as quantitative, for the notable time frame and for…
