Press release
Pre-Filled Syringes Market to Surpass $30B by 2033, Driven by Biologics Demand, Self-Administration, and Smart PFS Innovations | Gerresheimer AG, Schott AG, Stevanato Group, Terumo Corporation, Sanofi, Hikma Pharmaceuticals PLC, MedXL Inc
The Pre-Filled Syringes market was valued at US$ 8.66 billion in 2024 and is expected to grow significantly, reaching approximately US$ 30.04 billion by 2033. This represents a strong compound annual growth rate (CAGR) of 15.5% during the forecast period from 2025 to 2033.Download Sample Report: https://www.datamintelligence.com/download-sample/pre-filled-syringes-market?sg
Key Market Drivers:
✅ Rising Demand for Biologics
The growing use of biologics to treat chronic conditions such as cancer, rheumatoid arthritis, and autoimmune diseases is a major driver. Biologics are often administered via injection, and pre-filled syringes offer a safer and more convenient method compared to traditional vials and ampoules.
✅ Need for Improved Patient Compliance
Pre-filled syringes simplify the injection process and reduce preparation time, making them ideal for home use. This convenience increases treatment adherence, especially for patients requiring frequent or long-term injections.
✅ Reduced Risk of Contamination and Dosing Errors
Since pre-filled syringes come with a fixed drug dose and are ready-to-use, they minimize the risk of human error, contamination, and needlestick injuries. These safety advantages are prompting hospitals and clinics to transition to pre-filled formats.
✅ Technological Advancements in Syringe Design
Innovations such as dual-chamber syringes, auto-injectors, and needle safety systems are enhancing the usability and functionality of pre-filled syringes. These improvements are making them more appealing for both healthcare providers and patients.
✅ Growth of Point-of-Care and Self-Administration Trends
As healthcare systems worldwide shift toward decentralization and outpatient care, the demand for devices that support point-of-care treatment is rising. Pre-filled syringes enable easier self-injection, aligning well with the global push toward personalized and home-based healthcare.
Market Segments:
• By Product (Needle Free Prefilled Syringes, Needled Prefilled Syringes)
• By Material (Glass Prefilled Syringes, Plastic Prefilled Syringes)
• By Design (Single Chambered PFS, Dual Chambered PFS)
• By Application (Diabetes, Rheumatoid Arthritis, Ophthalmology, Anaphylaxis, Others)
• By End-User (Hospitals & Clinics, Ambulatory Surgical Centers, Others)
Make an Enquiry for purchasing this Report @ https://www.datamintelligence.com/enquiry/pre-filled-syringes-market?sg
Pre-Filled Syringes Market: Geographical Share
North America holds a significant share of the global pre-filled syringes market, driven by its highly developed healthcare infrastructure and increasing adoption of self-injection devices. The region benefits from strong regulatory frameworks that support the safety and standardization of injectable drug delivery systems. The United States, in particular, sees widespread use of biologics and biosimilars, further fueling market expansion.
Europe is another dominant region, with countries like Germany, France, and the United Kingdom leading the way. The region's mature pharmaceutical industry, along with increasing demand for home-based healthcare solutions, contributes to strong market growth. Additionally, European regulatory agencies encourage the use of pre-filled syringes due to their ability to minimize dosage errors and enhance patient compliance.
Asia-Pacific is expected to witness the fastest growth rate, thanks to rising healthcare expenditures, expanding access to biologics, and a growing geriatric population. Countries like China, India, and Japan are investing heavily in healthcare modernization and domestic pharmaceutical production, which is boosting the adoption of pre-filled syringe formats for chronic disease management.
DataM Intelligence Opinion:
The Pre-Filled Syringes Market is at a pivotal juncture, fueled by a confluence of clinical needs and technological advancements. With biologics gaining prominence in the treatment of chronic diseases like cancer and autoimmune conditions, the demand for efficient drug delivery systems is surging.
Pre-filled syringes offer a critical solution by enhancing patient compliance, reducing the risk of contamination, and simplifying self-administration key attributes in today's evolving healthcare landscape.
Innovations such as dual-chamber and needle-safe designs are further strengthening the market appeal, positioning pre-filled syringes as the preferred mode of injectable drug delivery for both providers and patients.
Geographically, the market showcases a balanced mix of maturity and high-growth potential. North America and Europe remain strongholds due to established healthcare infrastructures, rising use of biologics, and favorable regulatory environments that emphasize patient safety and product standardization.
However, the Asia-Pacific region is emerging as a high-growth hotspot, propelled by increasing healthcare investments, aging populations, and the rapid adoption of home-based care. As healthcare delivery continues to decentralize globally, DataM Intelligence believes that the strategic role of pre-filled syringes will only intensify redefining patient care through safety, convenience, and innovation.
Stay informed with the latest industry insights-start your subscription now: https://www.datamintelligence.com/reports-subscription?sg
Market Key Players:
Key players are BD, Gerresheimer AG, Schott AG, Stevanato Group S.p.A., Terumo Corporation, Sanofi S.A., Hikma Pharmaceuticals PLC, MedXL Inc., West Pharmaceutical Services, Inc., and Cardinal Health.
Key 2025 Developments in the Pre Filled Syringes Market:
Traceability Innovation: RFID‐Enabled Smart PFS:
Avery Dennison, in collaboration with Becton Dickinson (BD), unveiled an RFID‐embedded "smart" needle shield for pre‐filled syringes the BD iDFillTM system. This innovation at Pharmapack 2025 enables unit‐level traceability by embedding RFID tags into the needle shield while preserving the syringe barrel's design a notable leap for authentication and lifecycle tracking.
Manufacturing Expansion & Automation Investments:
Pharmaceutics International (Pii) is investing US $3.6 million in its prefilled syringe manufacturing capabilities including automated visual inspection tech from Antares and a syringe labeling line from Optima. These upgrades aim to be fully operational by mid‐to‐late 2025, enhancing fill/finish operations with greater automation and quality control.
M&A Activity & Facility Scaling:
Sharps Technology has struck a 5‐year, $200 million sales agreement with Nephron Pharmaceuticals, covering next‐generation copolymer PFS (10 mL, 50 mL) and SoloGard polypropylene syringes. Concurrently, Sharps is acquiring a state‐of‐the‐art prefillable syringe facility in South Carolina (US) for $35 million, expected to deliver initial product shipments by Q2 2025. This move positions it as the only fully dedicated COC prefillable syringe plant in North America.
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pre-Filled Syringes Market to Surpass $30B by 2033, Driven by Biologics Demand, Self-Administration, and Smart PFS Innovations | Gerresheimer AG, Schott AG, Stevanato Group, Terumo Corporation, Sanofi, Hikma Pharmaceuticals PLC, MedXL Inc here
News-ID: 4136614 • Views: …
More Releases from DataM Intelligence 4 Market Research LLP
Third-Party Logistics (3PL) Market Size, Share & Forecast 2024-2031 | Major Comp …
The Third-Party Logistics Market is estimated to reach growth at a Significant CAGR during the forecast period (2024-2031).
The Third-Party Logistics Market Market receives exhaustive analysis from DataM Intelligence, delivering stakeholders essential market data, emerging industry patterns, and strategic business intelligence. This in-depth research explores the competitive landscape in detail, evaluating market leaders across multiple dimensions including their innovative product offerings, competitive pricing strategies, financial performance metrics, strategic growth plans, and…
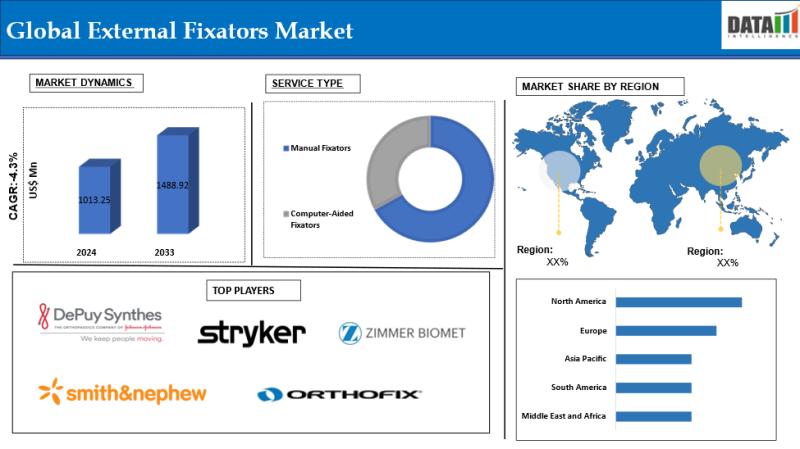
External Fixators Market to Surpass $1.48 Billion by 2033, Driven by Aging Popul …
The External Fixators market was valued at US$ 1,013.25 million in 2024 and is projected to reach approximately US$ 1,488.92 million by 2033, growing at a compound annual growth rate (CAGR) of 4.3% during the forecast period from 2025 to 2033.
Download your exclusive sample report today: (corporate email gets priority access): https://www.datamintelligence.com/download-sample/external-fixators-market?sg
Key Market Drivers:
• Rising Trauma and Accident Cases
A global increase in road traffic accidents, workplace injuries, and sports-related trauma is…
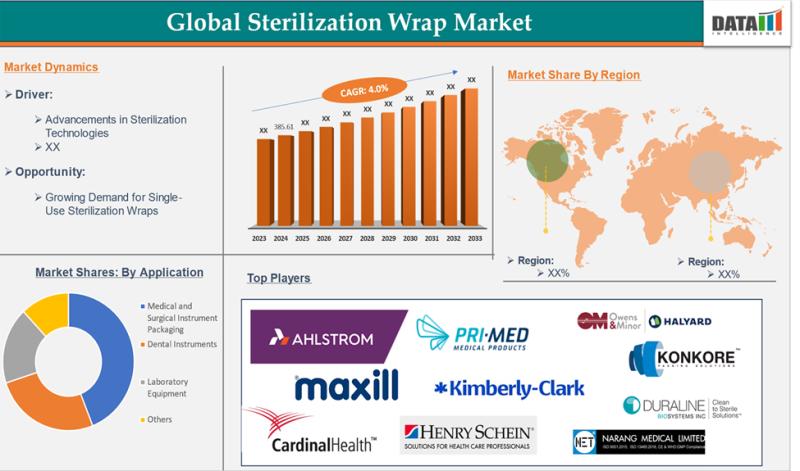
Sterilization Wrap Market to Hit $527.08M by 2033, Driven by Surgical Volume, In …
The Sterilization Wrap market was valued at US$ 385.61 million in 2024 and is expected to grow to approximately US$ 527.08 million by 2033, registering a compound annual growth rate (CAGR) of 4.03% during the forecast period from 2025 to 2033.
Download your exclusive sample report today: (corporate email gets priority access): https://www.datamintelligence.com/download-sample/sterilization-wrap-market?sg
Key Market Drivers:
• Rising Surgical Volumes
The global increase in elective and emergency surgical procedures is a major factor propelling demand.…
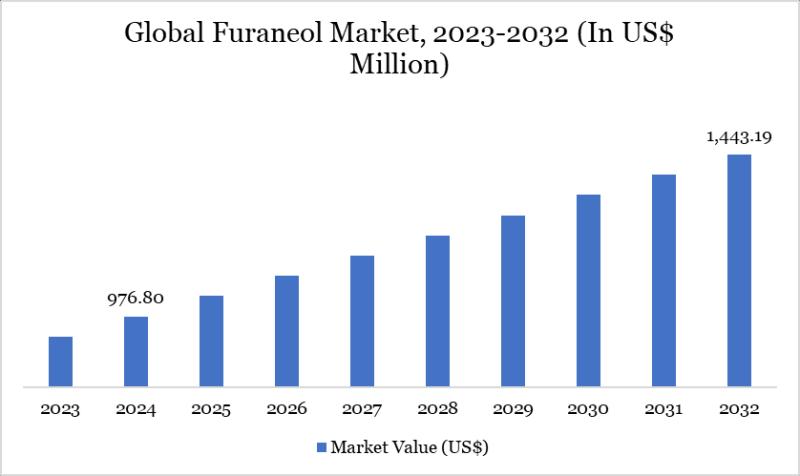
Furaneol Market Size & Share 2025-2032
Market Size and Growth
As per DataM Intelligence Experts Research Report Global furaneol market reached US$ 976.80 million in 2024 and is expected to reach US$ 1,443.19 million by 2032, growing at a CAGR of 5% during the forecast period 2025-2032.
Furaneol Market report, published by DataM Intelligence has released its latest in-depth analysis on the global Furaneol Market, delivering a detailed overview of regional growth patterns, market segmentation, CAGR, and financial…
More Releases for Pre
Pre-Shipment Inspection India, Pre-Shipment Inspection, PSI Certification, CE Ce …
Ravi Energie is a mandated PSI Agency and a Pre-shipment Inspection Certificate from Ravi Energie is required for Customs Clearance in the “Member Countries”. Once a pre shipment inspection entity and an exporter agree on an inspection date, the pre shipment inspection entity conducts the inspection on that date unless it is rescheduled on a mutually agreed basis between the exporter and the pre shipment inspection entity. Pre-shipment Inspection (PSI)…
PSI Certification, Pre Shipment Documents, CE Certification, Industrial Pre Ship …
Ravi Energie Inc has been providing multi-level and multi-disciplinary services related to International Trade and Energy since 1976. Broadly Categorizing, Ravi Energie has a unique global network and being an inspection agency that's just around the corner, we provide services at virtually every harbor and airport around the world. Ravi Energie understands each country's policy and accordingly checks for the implementation and gives a certification.
Ravi Energie provide a real competitive…
Pre-Shipment Inspection, Pre Shipment Certification, Certification, Pre Shipment …
Ravi energie Inc offers fast, expert product testing, inspection and certification solutions through our global network of accredited laboratories. Ravi energie Inc. supports manufacturers of energy generation and distribution equipment with testing and certification solutions for access to global markets. Whether you're engaged in fossil fuel or renewable energy development,
Ravi Energie which is an ISO 9001 certified company Customs classification is verified for each imported item to allow Customs…
Pre-Shipment Inspection Certification, Pre Shipment Verification, Pre Shipment C …
Ravi Energie also issues Clean Report of Findings (CRF) after inspection which specifying that the goods have been inspected before shipment and the price of the goods exported has been verified. In most PSI-using countries, physical inspection and price verification of almost all goods prior to exportation is obligatory for imports to be permitted today over 30 countries in Africa, Asia and Latin America use Pre-shipment Inspection Certification services.
In India…
Pre-Shipment Inspection Certification, Pre Shipment Certification, Pre Shipment …
Ravi Energie Provide Pre-Shipment Inspection Certification, Pre Shipment Documents as being mandated PSI Agency and a PSI Certificate from us is required for Customs Clearance in the Member Countries. Pre shipment inspection activities are all activities relating to the verification of the quality, quantity. In most PSI-using countries, physical inspection and price verification of almost all goods prior to exportation is obligatory for imports to be permitted today over 30…
CE Certification, Industrial Pre Shipment Inspection, PSI Certification, Pre Shi …
Ravi Energie is an ISO 9001 c`ertified USA & India based company and authorized to provide services like Pre-Shipment Inspection. Pre-shipment inspection includes the checking of the commodity against any list of items subject to import regulations. Customs classification is verified for each imported item to allow Customs to apply the correct tariff rates.
The fact of an import-driven economy made it essential to monitor the quantity and quality of goods…
