Press release
$40 Price Target in New H. C. Wainright Analyst Report on Leader in $3 Billion Suicidal Depression Market with Superior NRX 100 Drug Therapy: NRx Pharmaceuticals, Inc. (Nasdaq: NRXP)
Image: https://www.globalnewslines.com/uploads/2025/09/1757392285.jpg$NRXP Continues Expansion with Completion of Dura Medical Acquisition in Network of Interventional Psychiatry Clinics
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression With Suicidality; Assuming Coverage with Buy and $40 Price Target.
* Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
* FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
* Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
* Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
* $7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with Universal Capital, LLC.
* Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
Image: https://www.globalnewslines.com/uploads/2025/09/7bbf1539d726c189c0b399605a6d38b6.jpg
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
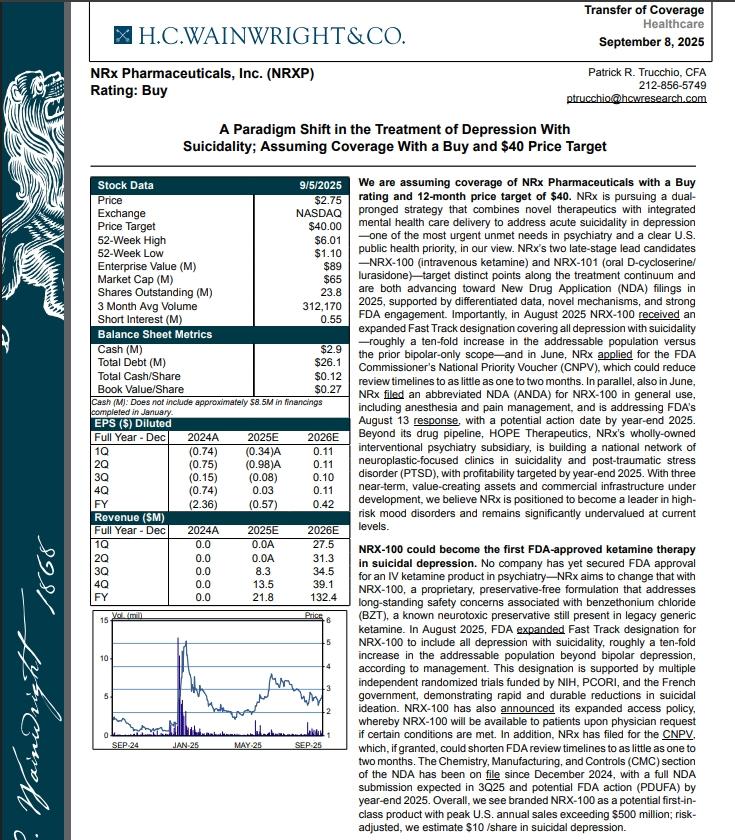
On September 8th H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Expanded Access Policy for NRX-100 (preservative-free ketamine)
On August 27th NRXP announced its expanded access policy for NRX-100 (preservative-free ketamine) based on grant of Fast Track [https://www.globenewswire.com/Tracker?data=Ji-EmYKuq9HHTbHjYkok6ifFWP0PphfpJK_ZW2Z5WWvGwtA2qARGrMYMc6slxJ0xc5SpzyOA04SHT3ksX3AVL8bnsiACsidx-RS8WIN2pP1vOf3g1VYCMDv4CRpM4UWfeVM_nwp2UcQGcPNY_4rJkQ41V73XdGEgAeZxrEJh_3DntKLR2tBELpfLd5pA-2BXzWFVa7b7jYQpgDWfvdNgbA==] designation for NRX-100 in the treatment of suicidal ideation in patients with depression, including bipolar depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet need, based on an assessment of the preliminary data contained in the Fast Track designation request. Accordingly, NRXP NRX-100 is available for expanded access to eligible patients.
Second Quarter 2025 Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Completion of a toxicology assessment of Benzethonium Chloride1, documenting its lack of "Generally Recognized as Safe" (GRAS) status and lack of safety data to support its use in intravenous presentations of ketamine.
NRXP filing of a Citizen's Petition with the U.S. Food and Drug Administration to seek the removal of benzethonium chloride, a toxic preservative, from all ketamine products for intravenous administration.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
HOPE Therapeutics
NRXP execution of definitive Purchase Agreement and receipt of final regulatory clearance from Florida's Agency for Health Care Administration ("ACHA") to proceed with closing the acquisition of Dura Medical.
Execution of binding letter of intent to acquire the assets of NeuroSpa TMS Holdings of Tampa, FL.
Execution of a binding letter of intent to acquire a 49% interest in Cohen and Associates, LLC.
NRXP Receipt of approval, pending legal stipulations, for $7.8 million of debt financing to support the acquisition of Dura Medical, NeuroSpa TMS Holdings, and Cohen and Associates, LLC.
Corporate (subsequent to the filing of form 10-Q)
NRXP $6.5 million dollar investment to purchase approximately 3.9 million shares of common stock of NRx Pharmaceuticals on August 18, 2025, by a consortium of experienced biotechnology investors led by B Group Capital. The purchase is subject to a one-year lockup on trading, shorting, or otherwise hypothecating said securities. The investment has no warrants, repricing provisions, commissions, or other structure.
The B Group Capital led consortium of ultra long-term healthcare specialist investors is highly strategic with extensive experience in complex clinical, regulatory, and commercial therapeutics but also direct ownership and management of multi-unit retail operations with potentially positive long-term implications for efforts to continue to scale and develop NRXP HOPE Therapeutics.
For more information on $NRXP visit: https://www.nrxpharma.com/ [about:blank] and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Media Contact
Company Name: NRx Pharmaceuticals, Inc.
Contact Person: Matthew Duffy, Chief Business Officer
Email: Send Email [http://www.universalpressrelease.com/?pr=40-price-target-in-new-h-c-wainright-analyst-report-on-leader-in-3-billion-suicidal-depression-market-with-superior-nrx-100-drug-therapy-nrx-pharmaceuticals-inc-nasdaq-nrxp]
Phone: 484 254 6134
Address:1201 Orange Street Suite 600
City: Miami
State: Florida
Country: United States
Website: https://www.nrxpharma.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. GetNews makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release $40 Price Target in New H. C. Wainright Analyst Report on Leader in $3 Billion Suicidal Depression Market with Superior NRX 100 Drug Therapy: NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) here
News-ID: 4176202 • Views: …
More Releases from Getnews

Federixa & BEAX release their electronic music
Image: https://www.globalnewslines.com/uploads/2025/12/5f95777dcc1e2dcc357a8a1d12fb3e49.jpg
Experimentation and creativity with over 100 official tracks at just 10 and 8 years old
Federixa (10 years old) and BEAX (8 years old) are two young Italian producers and songwriters with an overwhelming passion for electronic music. They don't sing, but they produce, write, and create original sounds ranging from Tech House to Melodic Techno, from Electro Dance to Electronic Hip Hop. Despite their young age, they are already…

brainSHARE Founder and Chamber Board Member Joe Siecinski Launches Complimentary …
Image: https://www.globalnewslines.com/uploads/2025/12/1765328151.jpg
"I can't stop the business ground from shifting. But through this program, I can help owners strengthen the systems and human connection they need to find their footing and move forward with confidence. That's why this matters now more than ever." - Joe Siecinski, Founder: brainShare Business Mentors.
brainSHARE Founder Joe Siecinski is offering new Silicon Valley Chamber members two months of free access to The Success Circle, helping business…

Otonomus Hotels Announces Global Expansion Following the Success of its Las Vega …
Las Vegas, NV - December 10, 2025 - Otonomus Hotels, recognized for launching what industry observers have called the world's first fully AI-powered hotel, is entering a significant global expansion phase following a strong debut in Las Vegas. The company has announced plans to open 40 new locations worldwide, bringing its technology-driven hospitality model to major destinations across the United States, Europe, Asia, and the Middle East.
Image: https://www.globalnewslines.com/uploads/2025/12/85650c1609eb28f4abc4997702a8d505.jpg
Launched early this…

Healing Through Exile: How Distance Becomes the Road Home Again
Image: https://www.globalnewslines.com/uploads/2025/12/1765400936.jpg
In its final movement, From Error to Error, Back and Forth transcends the confines of memoir and enters the realm of philosophical literature. Monika Killeen's M - now settled on a small island off the coast of New Zealand - reflects on freedom, belonging, and the fragile equilibrium of happiness.
Through encounters with new friends like the enigmatic Constance, and moments of startling clarity, M begins to understand that peace…
More Releases for NRX
Advancements on Multiple Fronts in $3 Billion Suicidal Depression Market, Highli …
Image: https://www.globalnewslines.com/uploads/2025/08/1755525181.jpg
$NRXP Sees 10-Fold Market Expansion to 13 Million Americans for Bipolar Depression Alone.
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
…
$3 Billion Suicidal Depression Market May Soon be Accessible via FDA Fast Track …
Image: https://www.globalnewslines.com/uploads/2025/08/1754917343.jpg
$NRXP Has $7.8 Million for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* FDA Fast Track Designation for NRX…
$300 Million in Milestones Plus Tiered Double-Digit Royalties from Accepted Term …
Image: https://www.globalnewslines.com/uploads/2025/03/1742217317.jpg
$NRXP is Poised to Address Over $3 Billion Suicidal Depression Market in the US
* Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
* Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
* Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
* New Drug Application for…
NRx Pharmaceuticals Highlights Breakthrough Oral Antidepressant's Efficacy in Re …
- Developing Therapeutics for the Treatment of CNS Disorders, Specifically Suicidal Bipolar Depression, Chronic Pain and PTSD.
- June Meeting of the American Society for Clinical Psychopharmacology Focused on Intravenous Ketamine and Intranasal S-Ketamine for Severe Depression and Suicidality.
- Presenters from 3 Open Label Studies at the ASCP Suggested Intravenous Ketamine is Equivalent or has Advantages over Intranasal S-Ketamine.
- NRXP Reached 9-Month Stability Point with its Ketamine Formulation (NRX-100) and Initiated…
Final Clinical Trial Results Show Superior Safety and Efficacy for NRX-101; Plan …
Developing Therapeutics for the Treatment of CNS Disorders, Specifically Suicidal Bipolar Depression, Chronic Pain and PTSD.
MOU Signed with Conversio Health with Immediate Plans to Ship IV Ketamine Product to Full Range of Customers via 503a and 503b Pharmacies.
Clinical Trial Success in Proving a Statistically-Significant 76% Reduction in Akathisia in Participants Treated with NRX-101 Compared to Lurasidone.
Company Plans to Seek Accelerated Approval of NRX-101 for Bipolar Depression and Akathisia and Broaden…
Complicated Urinary Tract Infections (UTI) Pipeline Analysis (2023) Covering Cli …
Las Vega (Nevada), United States //- As per DelveInsight's assessment, globally, about 12+ key pharma and biotech companies are working on 12+ pipeline drugs in the Complicated Urinary Tract Infections therapeutics landscape based on different Routes of Administration (ROA), Mechanism of Action (MOA), and molecule types. Several of the therapies are in the advanced stages of clinical development and are expected to launch in the coming years.
"Complicated Urinary Tract Infections…