Press release
Choroideremia Pipeline Assessment | In-depth Insights into the Emerging Drugs, Latest FDA, EMA, and PMDA Approvals, Clinical Trials, and Treatment Outlook | Curative Biotech, 4D Molecular Therapeutics
Las Vega (Nevada), United States //- As per DelveInsight's assessment, globally, Choroideremia pipeline constitutes key companies continuously working towards developing Choroideremia treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.The Choroideremia Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also covers a detailed description of the drug, including the mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, collaborations, mergers acquisition, funding, designations, and other product-related details.
"Choroideremia Pipeline Insight, 2024 [https://www.delveinsight.com/sample-request/choroideremia-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr]" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the Choroideremia Market.
Some of the key takeaways from the Choroideremia Pipeline Report:
* Companies across the globe are diligently working toward developing novel Choroideremia treatment therapies with a considerable amount of success over the years. Choroideremia Key players such as - Curative Biotech, 4D Molecular Therapeutics, Spark Therapeutics, NightstaRx Ltd., and others , are developing therapies for the Choroideremia treatment
* Choroideremia Emerging therapies such as - Metformin, 4D-110, SPK-7001, BIIB111, and others are expected to have a significant impact on the Choroideremia market in the coming years.
* In November 2023, Brian Strem, CEO of Kiora Pharmaceuticals, spoke with Steve Darling from Proactive to announce the company's strategy to expand the clinical exploration of KIO-301, a therapy aimed at treating different inherited retinal conditions. This expansion entails launching a controlled, double-masked, randomized Phase 2 trial with escalating doses.
Choroideremia Overview
Choroideremia is a rare genetic disorder of vision that is more common in man. It is an X-linked recessive condition that leads to gradual degradation of choroid, retinal pigment epithelium (RPE), and photoreceptors. Female are mostly the carriers and mostly unaffected, even so they can experience minor symptoms, such as blurred vision in later stages of their life
Get a Free Sample PDF Report to know more about Choroideremia Pipeline Therapeutic Assessment:
https://www.delveinsight.com/report-store/choroideremia-pipeline-insight [https://www.delveinsight.com/report-store/choroideremia-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr]
Route of Administration
Choroideremia pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs, such as
* Oral
* Parenteral
* Intravenous
* Subcutaneous
* Topical
Molecule Type
Products have been categorized under various Molecule types, such as
* Monoclonal Antibody
* Peptides
* Polymer
* Small molecule
* Gene therapy
Choroideremia Pipeline Therapeutics Assessment
* Choroideremia Assessment by Product Type
* Choroideremia By Stage and Product Type
* Choroideremia Assessment by Route of Administration
* Choroideremia By Stage and Route of Administration
* Choroideremia Assessment by Molecule Type
* Choroideremia by Stage and Molecule Type
DelveInsight's Choroideremia Report covers around products under different phases of clinical development like
* Late-stage products (Phase III)
* Mid-stage products (Phase II)
* Early-stage product (Phase I)
* Pre-clinical and Discovery stage candidates
* Discontinued & Inactive candidates
* Route of Administration
Further Choroideremia product details are provided in the report. Download the Choroideremia pipeline report to learn more about the emerging Choroideremia therapies [https://www.delveinsight.com/sample-request/choroideremia-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr]
Choroideremia Pipeline Analysis:
The Choroideremia pipeline report provides insights into
* The report provides detailed insights about companies that are developing therapies for the treatment of Choroideremia with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Choroideremia Treatment.
* Choroideremia key companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Choroideremia Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Choroideremia market.
The report is built using data and information traced from the researcher's proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations, and featured press releases from company/university websites and industry-specific third-party sources, etc.
Some of the key companies in the Choroideremia Therapeutics Market include:
Key companies developing therapies for Choroideremia are - NightstaRx Ltd/Biogen, 4D Molecular Therapeutics, Curative Biotech, Therapeutics, Spark therapeutics/Roche, and others.
Emerging Choroideremia Drugs Under Different Phases of Clinical Development Include:
* Metformin: Curative Biotech
* 4D-110: 4D Molecular Therapeutics
* SPK-7001: Spark Therapeutics
* BIIB111: NightstaRx Ltd.
Download Sample PDF Report to know more about Choroideremia drugs and therapies [https://www.delveinsight.com/sample-request/choroideremia-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr]
Scope of Choroideremia Pipeline Drug Insight
* Coverage: Global
* Key Choroideremia Companies: Curative Biotech, 4D Molecular Therapeutics, Spark Therapeutics, NightstaRx Ltd., and others
* Key Choroideremia Therapies: Metformin, 4D-110, SPK-7001, BIIB111, and others
* Choroideremia Therapeutic Assessment: Choroideremia current marketed and Choroideremia emerging therapies
* Choroideremia Market Dynamics: Choroideremia market drivers and Choroideremia market barriers
Request for Sample PDF Report for Choroideremia Pipeline Assessment and clinical trials [https://www.delveinsight.com/sample-request/choroideremia-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr]
Table of Contents
1
Choroideremia Report Introduction
2
Choroideremia Executive Summary
3
Choroideremia Overview
4
Choroideremia- Analytical Perspective In-depth Commercial Assessment
5
Choroideremia Pipeline Therapeutics
6
Choroideremia Late Stage Products (Phase II/III)
7
Choroideremia Mid Stage Products (Phase II)
8
Choroideremia Early Stage Products (Phase I)
9
Choroideremia Preclinical Stage Products
10
Choroideremia Therapeutics Assessment
11
Choroideremia Inactive Products
12
Company-University Collaborations (Licensing/Partnering) Analysis
13
Choroideremia Key Companies
14
Choroideremia Key Products
15
Choroideremia Unmet Needs
16
Choroideremia Market Drivers and Barriers
17
Choroideremia Future Perspectives and Conclusion
18
Choroideremia Analyst Views
19
Appendix
20
About DelveInsight
*The Table of Contents (TOC) is not exhaustive; the final content may vary. Refer to the sample report for the complete table of contents.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate business growth and overcome challenges with a practical approach.
Media Contact
Company Name: DelveInsight
Contact Person: Gaurav Bora
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=choroideremia-pipeline-assessment-indepth-insights-into-the-emerging-drugs-latest-fda-ema-and-pmda-approvals-clinical-trials-and-treatment-outlook-curative-biotech-4d-molecular-therapeutics]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Choroideremia Pipeline Assessment | In-depth Insights into the Emerging Drugs, Latest FDA, EMA, and PMDA Approvals, Clinical Trials, and Treatment Outlook | Curative Biotech, 4D Molecular Therapeutics here
News-ID: 3456193 • Views: …
More Releases from ABNewswire
Exploring the Impact of U.S. Government Shutdown on the Global Markets - An Excl …
Image: https://www.abnewswire.com/upload/2025/11/6ab92174e3dc0ee6c1bfafc8e655cfb4.jpg
A shutdown of the U.S. federal government, when Congress fails to pass spending legislation and many agencies cease non-essential operations, poses a material risk to the economy. But when viewed through the lens of market history and global asset flows, the evidence suggests that such shutdowns are often absorbed by the markets and, in several ways, may present tactical opportunities for globally diversified investors.
1. What a U.S. Government Shutdown…
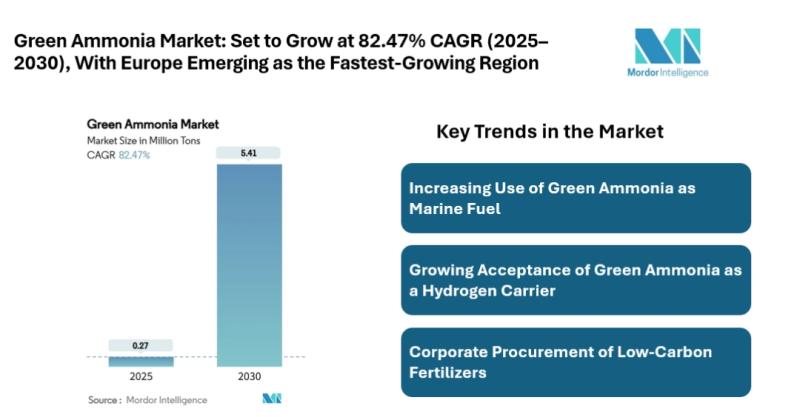
Green Ammonia Market size growing at CAGR of 82.47% by 2030 | Rising Fertilizer …
The latest research by Mordor Intelligence covers the "Green Ammonia Market," delivering insights into market dynamics, drivers of growth, and long-term forecasts.
Green Ammonia Market Overview:
The global green ammonia market is expanding steadily as countries accelerate the shift toward low-carbon fertilizer, marine fuel, power generation, and hydrogen transport solutions. The green ammonia market [https://www.mordorintelligence.com/industry-reports/green-ammonia-market?utm_source=abnewswire] size is expected to grow from 0.27 million tons in 2025 to 5.41 million tons by 2030,…
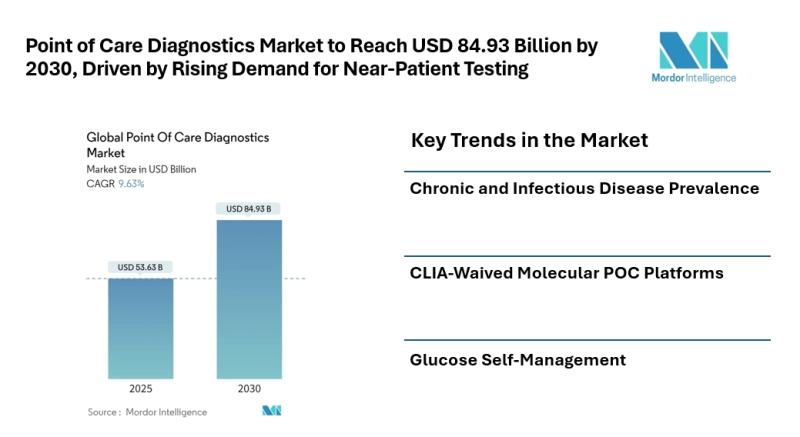
Point of Care Diagnostics Market to Reach USD 84.93 Billion by 2030, Driven by R …
Mordor Intelligence has published a new report on Point Of Care Diagnostics Market offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
The Point Of Care Diagnostics Market [https://www.mordorintelligence.com/industry-reports/point-of-care-diagnostics?utm_source=abnewswire] size is estimated at USD 53.63 billion in 2025, and is expected to reach USD 84.93 billion by 2030, at a CAGR of 9.63% during the forecast period (2025-2030). The market's growth is fueled by the increasing need for rapid,…
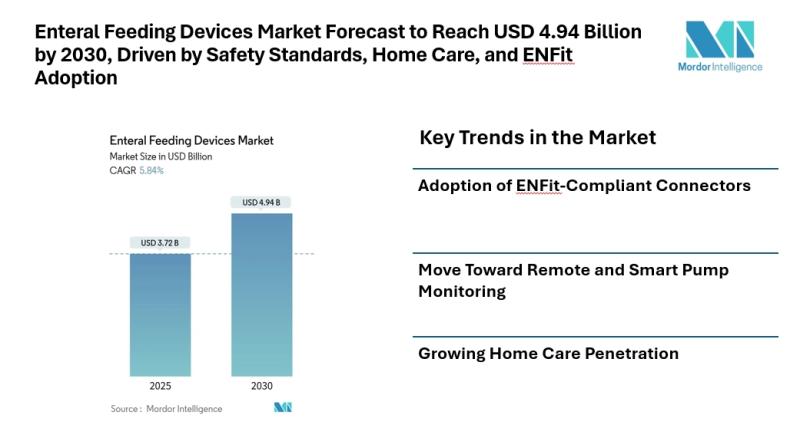
Enteral Feeding Devices Market Forecast to Reach USD 4.94 Billion by 2030, Drive …
Mordor Intelligence has published a new report on enteral feeding devices market offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
The enteral feeding devices market [https://www.mordorintelligence.com/industry-reports/enteral-feeding-devices-market?utm_source=abnewswire] is projected to grow from approximately USD 3.72 billion in 2025 to USD 4.94 billion by 2030, according to a recent report by Mordor Intelligence. The growth reflects a compound annual growth rate (CAGR) of around 5.84 percent, driven primarily by technological…
More Releases for Choroideremia
Choroideremia Market Set to Surge at 12.4% CAGR, Reaching USD 210 Million by 203 …
Introduction
Choroideremia is a rare, inherited X-linked retinal degenerative disease characterized by progressive vision loss, night blindness, and eventual blindness, typically in male patients.
Currently, no approved treatment exists to halt or reverse choroideremia progression. However, the emergence of gene therapies, stem cell therapies, and retinal implants is transforming the therapeutic outlook. With rising research investment, orphan drug designations, and growing patient advocacy, the Choroideremia Market is expected to expand significantly…
Choroideremia Market Size, Overview, Research Report & Forecast 2023-2033
Choroideremia Market Report Overview:
Report Attribute Details
Base Year 2022
Forecast Years 2023-2033
Historical Years …
Choroideremia Market Size, 2023 Analysis, Industry Trends and Forecasts to 2033
Choroideremia Market Report Overview:
Report Attribute Details
Base Year 2022
Forecast Years …
Choroideremia Treatment Market Growth Prospects, Trends and Forecast by 2028
The choroideremia treatment market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses the market to account to USD 4306.19 million by 2028 and will grow at a CAGR of 6.28% in the above mentioned forecast period.
Global Choroideremia Treatment Market Scope and Market Size
The choroideremia treatment market is segmented on the basis of therapy type, route of administration and distribution…
Choroideremia Treatment Market Trends, Revenue, Major Players, Share Analysis & …
Global Choroideremia Treatment market survey report documented is a rolling dice for the competitors to design strategies and become slightly more decisive to accomplish more profitability by prioritizing the vision of the organization. For structuring the finest market research report like Choroideremia Treatment, a devoted team of experienced forecasters, well-versed analysts, and knowledgeable researchers work painstakingly. The persuasive market report estimates the growth rate and the market value based on…
Choroideremia Treatment Market Size And Forecast - 2023-2031 | Biogen Inc., Wize …
Newark, New Castle, USA - The latest report from Growth Plus Reports analyzes the production, potential applications, demand, major manufacturers, and SWOT analysis of the global Choroideremia Treatment Market.
The Choroideremia Treatment Market Report assists in determining the optimum distribution methods for certain products as well as possible markets for future product launches. The report also analyses the purchase and supply trends that influence the market's production strategy. The Choroideremia Treatment…
