Press release
MAIA Biotechnology (MAIA): Pioneering New Frontiers in NSCLC Treatment with THIO
All exceptional measures of efficacy in our trial to date have exceeded our own expectations and outperformed standard of care treatments," said Vlad Vitoc, M.D., MAIA's Chairman and Chief Executive Officer. "The data presented at ASCO advances THIO's excellent clinical profile as a strong, safe, and highly effective alternative for patients who progressed following chemotherapy and other available treatments. We eagerly anticipate full efficacy data from THIO-101 in the second half of yearIn a significant development within the oncology landscape, MAIA Biotechnology, Inc. (NYSE American: MAIA), a trailblazer in clinical-stage biopharmaceuticals focusing on targeted immunotherapies for cancer, recently unveiled compelling efficacy data from its Phase 2 THIO-101 clinical trial. This trial critically evaluates THIO, a pioneering telomere-targeting agent, in sequence with cemiplimab (Libtayo Registered ) for advanced non-small cell lung cancer (NSCLC) patients who have not responded to two or more standard therapies.
The latest findings, which were presented at the American Society of Clinical Oncology (ASCO) 2024 Annual Meeting, show a remarkable overall response rate (ORR) of 38% and a disease control rate (DCR) of 85% when THIO is paired with the immune checkpoint inhibitor cemiplimab in third-line treatment scenarios. These results substantially outperform the typical 25-35% DCR observed with standard chemotherapy regimens, signaling a significant breakthrough in the treatment of this challenging cancer type.
Dr. Vlad Vitoc, Chairman and Chief Executive Officer of MAIA, expressed his enthusiasm about the results, stating, "All exceptional measures of efficacy in our trial to date have exceeded our own expectations and outperformed standard of care treatments." He added that the data "advances THIO's clinical profile as a strong, safe, and highly effective alternative for patients who progressed following chemotherapy and other available treatments."
Key Results from THIO-101 Phase 2 Trial
The primary objectives of the THIO-101 trial are to assess the safety and tolerability of THIO and its efficacy in terms of ORR. To date, THIO, combined with cemiplimab, has demonstrated impressive tolerability in a heavily pre-treated patient population, with full enrollment completed ahead of schedule.
Significant findings include:
- An 85% DCR for THIO compared to the standard chemotherapy DCR of 25-35%.
- A 65% crossing of the 5.8-month overall survival (OS) threshold.
- A median progression-free survival (PFS) of 5.5 months at the optimal THIO dose of 180mg, with 88% of patients surpassing the 2.5-month PFS threshold.
THIO and Its Clinical Impact
THIO (6-thio-2'-deoxyguanosine) is at the forefront of telomere-targeting approaches in cancer therapy. It works by inducing telomerase-dependent DNA damage in cancer cells, triggering immune responses that lead to selective cancer cell death. This innovative mechanism not only disrupts the fundamental survival pathways of cancer cells but also enhances the effectiveness of subsequent immunotherapy treatments.
Looking Forward
With the anticipation of full efficacy data in the latter half of the year, MAIA Biotechnology is poised to redefine treatment paradigms in NSCLC. The company also expects THIO-101 to be the first completed clinical study of a telomere targeting agent, marking a pivotal milestone in cancer drug discovery and treatment. For more detailed information and ongoing updates, investors and stakeholders are encouraged to visit the poster session details and other company presentations available on MAIA's website at www.maiabiotech.com. As MAIA Biotechnology continues to advance its clinical trials and share promising results, the potential for THIO as a transformative treatment for NSCLC looks increasingly promising. This not only represents a significant stride forward for the company but also offers new hope for patients battling advanced stages of non-small cell lung cancer.
For traders and investors, MAIA's progress in clinical developments and the robust performance of THIO in trials suggest a promising horizon for stock valuation, especially considering the unmet medical needs in the NSCLC market. As such, MAIA represents a potentially valuable addition to investment portfolios focused on high-impact biotechnology sectors.
Other biotech stocks to keep on top of radar include Biomarin Pharmaceuticals Inc. (NASDAQ: BMRN), Halozyme Therapeutics, Inc. (NASDAQ: HALO), Incyte Corporation (NASDAQ: INCY), Exelixis, Inc. (NASDAQ: EXEL), Mimedx Group, Inc. (NASDAQ: MDXG), Genmab AS (NASDAQ: GMAB), Vertex Pharmaceuticals Inc. (NASDAQ: VRTX), Bio-Techne Corp (NASDAQ: TECH), Jazz Pharmaceuticals PLC (NASDAQ: JAZZ).
Disclaimer: This blog post is for informational purposes only and does not constitute financial advice or an endorsement of MAIA or its strategies. FOR EDUCATIONAL AND INFORMATION PURPOSES ONLY; NOT INVESTMENT ADVICE. Please ensure to fully read and comprehend our disclaimer found at https://investorbrandmedia.com/disclaimer/. InvestorBrandMedia.com has been compensated five hundred dollars by a 3rd party Momentum Media LLC for content distribution services on MAIA for June 7th, 2025. We own zero shares of MAIA. InvestorBrandMedia.com is neither an investment advisor nor a registered broker. No current owner, employee, or independent contractor of InvestorBrandMedia.com is registered as a securities broker-dealer, broker, investment advisor, or IA representative with the U.S. Securities and Exchange Commission, any state securities regulatory authority, or any self-regulatory organization. This article may contain forward-looking statements as defined under Section 27A of the Securities Act of 1933 and 21E of the Exchange Act of 1934. These statements, often incorporating terms like "believes," "anticipates," "estimates," "expects," "projects," "intends," or similar expressions about future performance or conduct, are based on present expectations, estimates, and projections, and are not historical facts. They carry various risks and uncertainties that may result in significant deviation from the anticipated results or events. Past performance does not guarantee future results.InvestorBrandMedia.com does not commit to updating forward-looking statements based on new information or future events. Readers are encouraged to review all public SEC filings made by the profiled companies at https://www.sec.gov/edgar/searchedgar/companysearch. It is always important to conduct thorough due diligence and exercise caution in trading.InvestorBrandMedia.com is not managed by a licensed broker, a dealer, or a registered investment adviser. The content here is purely informational and should not be taken as investment advice. The Private Securities Litigation Reform Act of 1995 provides investors a safe harbor regarding forward-looking statements. Any statement that projects, foresees, expects, anticipates, estimates, believes, or understands certain actions to possibly occur are not historical facts and may be forward-looking statements. These statements are based on expectations, estimates, and projections that could cause actual results to differ greatly from those anticipated. Investing in micro-cap and growth securities is speculative and entails a high degree of risk, potentially leading to a total or substantial loss of investment. Please note that no content published here constitutes a recommendation to buy or sell a security. It is solely informational, and you should not construe it as legal, tax, investment, financial, or other advice. No content in this article constitutes an offer or solicitation by InvestorBrandMedia.com or any third-party service provider to buy or sell securities or other financial instruments. The content in this article does not address the circumstances of any specific individual or entity and does not constitute professional and/or financial advice. InvestorBrandMedia.com is not a fiduciary by virtue of any person's use of or access to this content.
Sources:
https://finance.yahoo.com/news/latest-global-non-small-cell-073000144.html
https://finance.yahoo.com/news/maia-biotechnology-reveals-clinical-data-124100573.html [https://finance.yahoo.com/news/maia-biotechnology-reveals-clinical-.html]
Media Contact
Company Name: Investor Brand Media
Contact Person: Ash K
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=maia-biotechnology-maia-pioneering-new-frontiers-in-nsclc-treatment-with-thio]
Country: United States
Website: https://investorbrandmedia.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release MAIA Biotechnology (MAIA): Pioneering New Frontiers in NSCLC Treatment with THIO here
News-ID: 3530369 • Views: …
More Releases from ABNewswire
Exploring the Impact of U.S. Government Shutdown on the Global Markets - An Excl …
Image: https://www.abnewswire.com/upload/2025/11/6ab92174e3dc0ee6c1bfafc8e655cfb4.jpg
A shutdown of the U.S. federal government, when Congress fails to pass spending legislation and many agencies cease non-essential operations, poses a material risk to the economy. But when viewed through the lens of market history and global asset flows, the evidence suggests that such shutdowns are often absorbed by the markets and, in several ways, may present tactical opportunities for globally diversified investors.
1. What a U.S. Government Shutdown…
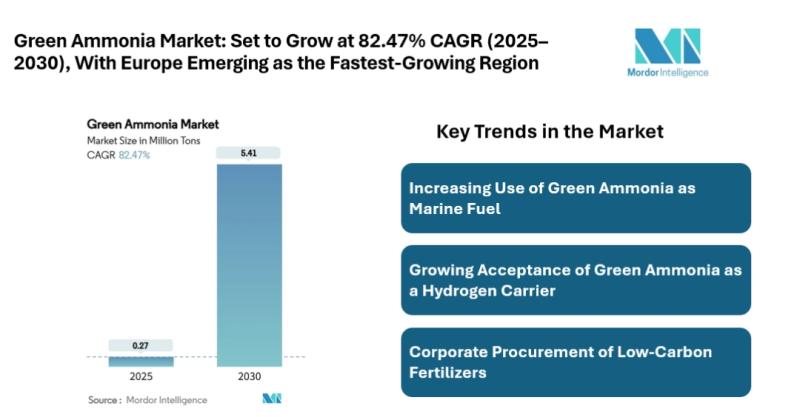
Green Ammonia Market size growing at CAGR of 82.47% by 2030 | Rising Fertilizer …
The latest research by Mordor Intelligence covers the "Green Ammonia Market," delivering insights into market dynamics, drivers of growth, and long-term forecasts.
Green Ammonia Market Overview:
The global green ammonia market is expanding steadily as countries accelerate the shift toward low-carbon fertilizer, marine fuel, power generation, and hydrogen transport solutions. The green ammonia market [https://www.mordorintelligence.com/industry-reports/green-ammonia-market?utm_source=abnewswire] size is expected to grow from 0.27 million tons in 2025 to 5.41 million tons by 2030,…
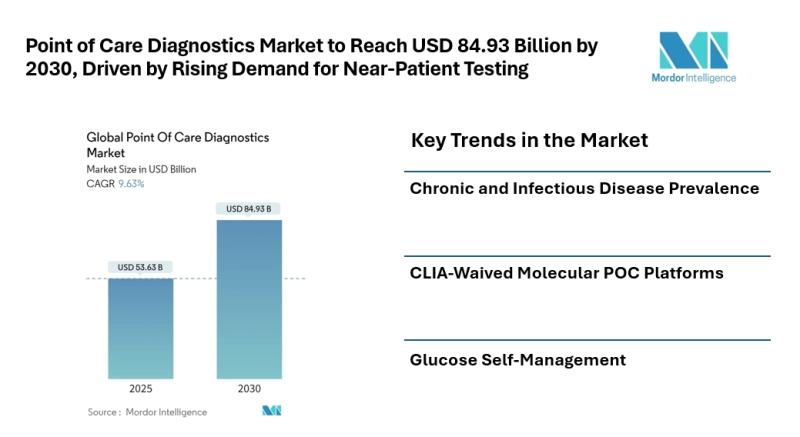
Point of Care Diagnostics Market to Reach USD 84.93 Billion by 2030, Driven by R …
Mordor Intelligence has published a new report on Point Of Care Diagnostics Market offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
The Point Of Care Diagnostics Market [https://www.mordorintelligence.com/industry-reports/point-of-care-diagnostics?utm_source=abnewswire] size is estimated at USD 53.63 billion in 2025, and is expected to reach USD 84.93 billion by 2030, at a CAGR of 9.63% during the forecast period (2025-2030). The market's growth is fueled by the increasing need for rapid,…
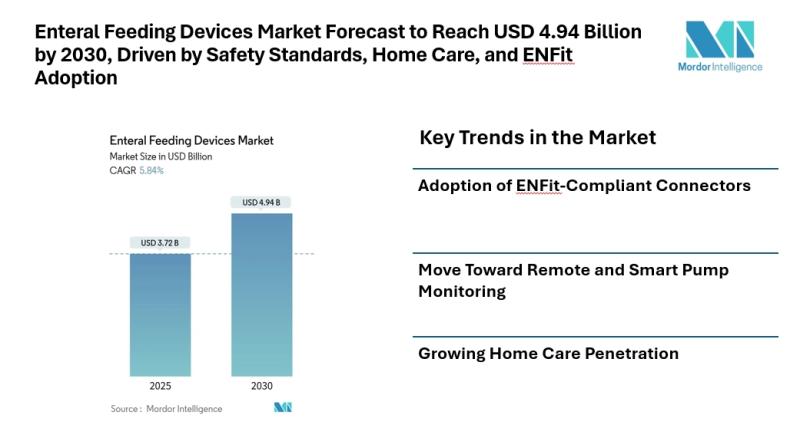
Enteral Feeding Devices Market Forecast to Reach USD 4.94 Billion by 2030, Drive …
Mordor Intelligence has published a new report on enteral feeding devices market offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
The enteral feeding devices market [https://www.mordorintelligence.com/industry-reports/enteral-feeding-devices-market?utm_source=abnewswire] is projected to grow from approximately USD 3.72 billion in 2025 to USD 4.94 billion by 2030, according to a recent report by Mordor Intelligence. The growth reflects a compound annual growth rate (CAGR) of around 5.84 percent, driven primarily by technological…
More Releases for MAIA
MAIA Biotechnology (MAIA) is one of the earliest pioneers of telomere targeting …
MAIA is one of the earliest pioneers of telomere targeting as a therapeutic strategy, and we share in the enthusiasm for the FDA approval of imetelstat for rare blood cancers originating in bone marrow. We have found that telomere targeting as a mechanism of action plays a key role in treating certain cancers, and we are studying this science in our Phase 2 trial of THIO in high-risk non-small cell…
Groundbreaking Study by MAIA Biotechnology (NYSE: MAIA) Indicates Promising New …
The NSCLC drug market is driven by advancements in targeted therapies, such as tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs), which offer improved efficacy and tolerability compared to traditional chemotherapy.
According to the latest research study [https://finance.yahoo.com/news/latest-global-non-small-cell-073000144.html], the demand of global Non-Small-Cell Lung Cancer Drug Market size & share was valued at approximately USD 31,614.8 Million in 2023 and is expected to reach USD 34,175.6 Million in 2024 and…
MAIA Biotechnology (MAIA) to Showcase Groundbreaking Telomere-Targeting Cancer T …
MAIA Chairman and CEO Vlad Vitoc, M.D. will deliver a presentation featuring up-to-date findings from the Company's Phase 2 THIO-101 clinical trial evaluating THIO sequenced with the immune checkpoint inhibitor (CPI) cemiplimab (Libtayo Registered ) in advanced non-small cell lung cancer (NSCLC).
MAIA Biotechnology, Inc. (NYSE American: MAIA) is capturing the attention of traders and investors with its innovative approaches to cancer treatment, focusing on telomere-targeting therapies. The company is scheduled…
MAIA Biotechnology Inc (MAIA) Stock Surges as Company Prepares to Present Promis …
Dr. Vitoc's presentation is scheduled for Wednesday, June 5 at 11:30 a.m. PDT in Company Presentation Theater 1, located in Hall A of the San Diego Convention Center. Dr. Vitoc and team members will host meetings with pharmaceutical executives, researchers, and others throughout the three-day event. To schedule a meeting, please submit a meeting request through the BIO One-on-One Partnering Trademark platform at the Convention website.
Biotechnology stocks represent some of…
MAIA Biotechnology (NYSE: MAIA) Makes Strategic Moves: Key Presentations, New Fu …
Biotech Stocks In Focus MAIA Biotechnology (NYSE: MAIA), Eli Lilly and Company (LLY), Novo Nordisk A/S (NVO), Johnson & Johnson (JNJ), Merck & Co., Inc. (MRK), AbbVie Inc. (ABBV)
MAIA Biotechnology Inc. (NYSE: MAIA) has been making waves in the biotech industry with several recent announcements that have garnered attention from traders and investors alike. These developments highlight the company's ongoing commitment to advancing cancer treatment and its potential as a…
Fusion Maia Da Nang Launch in Singapore
“Why didn't anyone think of this before? The Fusion Maia resort in Da Nang, Vietnam, is the first five-star hotel in Asia with unlimited spa treatments included in the regular room rate. And by regular room, we mean, at minimum, a 50-sq-m suite with a private pool.” - Jeninne Lee-St. John, www.time.com
Between 6 April and 8 April Fusion Maia Da Nang will organize a Launch in Singapore to introduce…
